Article Text
Abstract
Background Diabetic foot ulceration (DFU) has devastating complications and a lifetime occurrence of 15%–34%. Debridement of DFU is regarded as an intervention that accelerates ulcer healing and may reduce complications including amputations, infections, and poor quality of life (QoL), which have serious public health and clinical implications. A systematic review (SR) of SRs and of randomized controlled trials (RCTs) with meta-analyses (MAs) on debridement of DFU that synthesizes all human experimental evidence is warranted.
Objectives Are debridement methods in DFU beneficial over other forms and standard gauze dressings (control condition) in these outcomes?
Study eligibility criteria All SRs/MAs/RCTs comparing debridement methods for DFU with alternative methods of debridement and with control.
Data sources Cochrane Wounds Group Specialized Register, Cochrane Central Register of Controlled Trials (Cochrane Library), Ovid MEDLINE, PubMed, EMBASE, EBSCO, CINAHL, and Web of Science.
Participants and interventions Adults with type 1/2 diabetes with DFU and any debridement method compared with alternative debridement methods or control.
Main Outcomes Amputation rates, wound infections, QoL, proportion of ulcers healed, time to complete healing, ulcer recurrence, and treatment cost.
Study selection and analysis Data extraction/synthesis by two independent reviewers pooled using a random-effects model with sensitivity analysis.
Results 10 SRs were retrieved and reported qualitatively. Six SRs included MAs. This SR included 30 studies, with 2654 participants, using 19 debridement combinations. The debridement methods were compared with findings pooled into MAs. Meta-regression (MR) did not identify significant predictors/moderators of outcomes.
Limitations The studies may have been under-powered. The inclusion/exclusion criteria varied and the increased risk of bias contributed to low-quality evidence.
Discussion/Conclusion Weak evidence exists that debridement methods are superior to other forms of debridement or control in DFU.
Implications Researchers should follow standardized reporting guidelines (Consolidated Standards of Reporting Trials). Clinicians/investigators could use the findings from this SR/MA/MR in guiding patient-individualized decision making and designing future RCTs.
- Outcomes Research
- Health Services Research
- Outcome Assessment (Health Care)
- Methodology
- Health Care Quality, Access, and Evaluation
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Outcomes Research
- Health Services Research
- Outcome Assessment (Health Care)
- Methodology
- Health Care Quality, Access, and Evaluation
What is already known about this subject?
Debridement of diabetic foot ulceration is a widely used method to remove devitalized tissue, although the ideal method(s) for debridement remain(s) unclear despite prior investigations.
These ulcerations place patients with diabetes at higher risk of infections, amputations, and disability, resulting in increased morbidity and premature mortality.
What are the new findings?
This systematic review (SR) is restricted to all human experimental evidence and includes 30 randomized controlled trials, synthesizing this body of evidence and evaluating the quality of evidence for risk of bias.
This SR found weak evidence that a form of debridement was superior to other forms or to a simple control condition using moistened gauze as an autolytic debridement, with the studies demonstrating significantly increased risk of bias.
How might these results affect future research or surgical practice?
Patients, healthcare providers, policy makers, and other stakeholders are careful in altering clinical practice on the basis of findings derived from small trials with unclear or high risk of bias, including non-randomized studies or weakly designed randomized studies.
Practitioners may choose to consider other characteristics such as individualization of therapy, patient tolerability, indications/contraindications, and cost when choosing between alternative methods of debridement.
Researchers should consider following the Consolidated Standards of Reporting Trials guidelines in an effort to improve reporting standards across all human experimental studies.
Background
Diabetic foot ulceration (DFU) has devastating complications, including amputations, poor quality of life (QoL), and serious complicating infections including osteomyelitis and sepsis.1–3 Diabetic wounds can be protracted and recur after healing (40% within 1 year and 65% within 3 years), consuming healthcare resources.3–5 These consequences have serious public health and clinical implications. Debridement is regarded as the standard of care that may help avoid these consequences. Debridement may include non-mechanical (autolytic and enzymatic) and mechanical (sharp/surgical, wet to dry, aqueous high-pressure lavage or irrigation, ultrasonic debridement, and biosurgery/maggot debridement therapy) methods. Debridement is used to remove non-viable/necrotic tissue in order to facilitate the wound healing process and help prevent disabling and fatal outcomes.6–8 The most effective method(s) of debridement remain(s) unclear. This systematic review on debridement of DFU retrieves and synthesizes all systematic reviews (SRs), meta-analyses (MAs), and randomized controlled trials (RCTs) in an effort to help answer this important research question.
DFU affects 15%–34% of patients with diabetes in their lifetime.9 10 The prevalence of DFU has been estimated to be 7% (4.8 million) in the UK and 9% (30.3 million) in the USA, and includes 7% (366 million) of the world’s population, with data trends suggesting rising rates.2 3 10 11 DFU increases the risk of amputations and complicating infections and reduces QoL. Debridement has been regarded as an effective intervention to help accelerate ulcer healing and may help reduce the risk of complications.12 13
Current published literature is unclear on which specific method of debridement ha beneficial effects and which has important public health and clinical implications, including amputation rates, complicating infection rates, QoL, wound healing and recurrence rates, time to complete healing, and costs.
SRs and MAs are critical tools in evidence-based medicine and healthcare.14 15 They provide researchers with an exhaustive, objective, scientific approach to synthesizing the evidence around a specific research question.16 A SR is a scientific investigation that is comprehensive and transparent and promotes duplication of effort, facilitating replication.14 15 This may include a qualitative SR and a quantitative SR (MA). SRs incorporate the use of objective search methods, data extraction tools, and risk of bias evaluation tools to help assess and judge the quality of evidence across all RCTs.14 15
Researchers can determine if a quantitative SR (MA) is warranted based on the results of a qualitative SR.16 A prerequisite to any planned SR is an exhaustive systematic search of the literature for other SRs conducted on similar research questions. SRs may include randomized and/or non-randomized trials.15–17
The studies captured in SRs may have included variable effect estimates which may have been measured on differing scales. Researchers can transform the different effect estimates into a common scale effect estimate.18 The effect estimates may include mean difference (MD), odds ratio (OR), relative risk (RR) ratio, standardized mean difference (SMD), or correlation coefficients.14 15
Analyzing prognostic risk factors can facilitate the development of population-specific guidelines and recommendations on the effects of debridement.
Methods
A literature search was designed to capture all RCTs, SRs, and MAs pertaining to the research question in an effort to synthesize all human experimental evidence. Data sources were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement Reporting guidelines.19
Study eligibility criteria
The following were the inclusion/exclusion criteria used to retrieve all prior SRs, MAs, and RCTs on the research question:
Type of studies
All SRs, MAs, and RCTs on debridement of DFU were included.
SRs/MAs/RCTs that were specific to wound types other than DFU or that included other wound types, that is, venous stasis ulcers, arterial insufficiency ulcers in patients without diabetes, pressure ulcers, and atypical ulcers, in the same sample were excluded. Atypical ulcer is defined as any and all ulcers/wounds not falling under the heading of DFU, pressure ulcer, venous stasis ulcer, or ischemic ulcer (due to peripheral arterial disease [PAD] in patients without diabetes). Atypical ulcers may include acute traumatic wounds, cancer-related wounds, and vasculitis-related wounds.
The search included any form of debridement but did not include SRs/MAs/RCTs on negative pressure wound therapy (NPWT). NPWT has been studied in separate SRs and has a multi-functional role beyond debridement and is not regarded as a primary form of debridement.20
All RCTs and SRs with and without MA, published or unpublished, were included in the search comparing the effectiveness of two or more methods of debridement or against the control condition in the treatment of DFU.
There were no restrictions based on language, country of origin, time period, healthcare setting, or publication status.
Non-randomized studies were excluded. The practice of combining randomized studies and non-randomized studies is generally discouraged. The practice of combining similarly designed studies in separate SRs, that is, randomized studies alone or non-randomized studies alone, is instead recommended.15–17 Non-randomized studies may be at higher risk of demonstrating biased and exaggerated effect estimates than randomized studies.15 17 21
Participants and interventions
Participants
Patients with type 1 or 2 diabetes with active DFU (neuropathic, neuroischemic, or ischemic etiology) and ≥18 years of age were included. The wounds were not limited in severity or in the grading system used. The grading systems included the Wagner Wound Grade and the Texas classification system.22 23 There were no other limitations, including gender. Studies that included wound types other than DFU in the same sample were excluded, unless a subgroup analysis was possible such that the researchers of this review could isolate the effects specific to patients with diabetes and DFU.
Interventions
Comparison of any method of debridement (removal of non-viable tissue from the wound) by either mechanical or non-mechanical debridement means as compared with the control condition or as compared with an alternative method of debridement was conducted in this SR/MA (online supplemental appendix table 1).
Supplemental material
Control condition
A simplified form of autolytic debridement which included saline and/or antiseptic solution-moistened gauze served as the control condition in this SR/MA.8
Outcome measures
Amputation frequency.
Complicating wound infection frequency including Methicillin resistant Staphylococcus aureus (MRSA).
QoL.
Healing rates.
Time to complete healing.
Recurrence rates.
Cost of care.
Primary outcomes
The primary outcomes included amputation frequency, complicating wound infection frequency (including MRSA), and QoL.
Secondary outcomes
The secondary outcomes included healing rates, time to complete healing, recurrent rates, and cost of care.
Data sources
Search methods for identification of studies
Three separate independent searches were conducted (see online supplemental appendix 2). These included searches done by the trial search coordinators at the Cochrane Review Group - Wounds (March 2013 and March 2015) and a separate institutional search by the trials search coordinator at the University of Connecticut research library (April 2014).
Supplemental material
Six computer databases were searched, including the WHO and conference proceedings and abstracts relevant to wound care (International Working Group on the Diabetic Foot, the European Wound Management Association, the American Professional Wound Care Association, and the European Tissue Repair Society).14 24–26 All of these searches were restricted to human experimental studies (RCTs) and all existing SRs/MAs related to the research question.27 Hand searching was conducted and included conference proceedings and journals not indexed in the electronic databases.
Manufacturers and distributors of debridement products were contacted for details of unpublished and ongoing trials. Experts in the field of DFU management were also contacted for details of unpublished and ongoing trials.
Studies accepted by the two independent reviewers using singular a priori inclusion/exclusion criteria were retrieved. These included studies where eligibility was not determined by the two reviewers based solely on title/abstract alone. Studies were retrieved in full text and scrutinized further if not excluded earlier based on title/abstract alone.
Electronic searches
Data sources were searched and collected and were reported following the PRISMA Statement Reporting guidelines19 (online supplemental appendix 2).
The following electronic databases were searched to find relevant studies:
Cochrane Wounds Group Specialized Register (15 April 2015).
Cochrane Central Register of Controlled Trials (1898–present).
Ovid MEDLINE (from 1996 to week 4 of March 2013).
Ovid MEDLINE (In-Process & Other Non-Indexed Citations) (1946–present, from 2013 to 14 April 2015).
Ovid EMBASE (1974–2015, from 16 June 1996 to week 13 of 2013, from 2013 to 14 April 2015).
EBSCO CINAHL (1981–present, from 2013 to 15 April 2015).
EMBASE via Scopus (1960–present).
Web of Science (1974–present).
Search in other resources
Bibliographies and citations were searched for all included SRs/MAs/RCTs related to the research question.
Selection of studies
On mutual consensus between the two reviewers, studies were then accepted for inclusion in this SR/MA (see online supplemental appendix 3A). Any discordance was resolved through discussion between the two reviewers, and if the discordance remained unresolved this was referred to a five-member panel of independent experts. The panel included two physicians, one statistician/meta-analyst, and two epidemiologists. Four out of the five members were associated with the University of Connecticut. The remaining panel member was a content expert physician from an external hospital institution.
Supplemental material
Any studies requiring full article retrieval that were subsequently rejected were indexed in a section for excluded studies, along with the specific reasons for rejection (online supplemental appendix 3B).
Supplemental material
Data collection and analysis
A coding system using a standardized data extraction form was developed and pilot-tested for use with all the variables selected for coding. The coding data extraction form included study-specific characteristics, quality-specific characteristics, participant-specific characteristics, and intervention-specific characteristics. These are further described.
Data extraction and management
Two independent reviewers independently extracted all SRs that met the predetermined inclusion criteria. Data from prior SRs were extracted along with the respective authors’ conclusions for comparison with this SR/MA using a data extraction tool developed for SRs/MAs.
The quantitative evidence from the included prior SRs was not pooled together in an MA for this review. This was due to significant variability in the 10 SRs retrieved. There was significant variability in (1) the inclusion/exclusion criteria used; (2) the study types retrieved, including combining both randomized and non-randomized studies; (3) the outcomes reported; and (4) the types of SRs retrieved (table 1).
Comparison of systematic reviews on debridement of diabetic foot ulcers
A data extraction tool was used to facilitate the qualitative review of all prior SRs retrieved for contrast purposes in this SR of all RCTs. The retrieved bibliographies of the SRs were hand-searched to locate additional SRs/MAs.
The data extracted from the SRs, if reported by the investigators of the RCTs retrieved, included the following:
Author/year (served as the study identification).
Number of studies included in the SR.
Study type, including randomized and non-randomized, and the number of studies for each designated SR.
Total number of participants included.
Follow-up period.
Study period.
Wound severity or grade.
Debridement intervention type.
Outcomes included in the SR.
Identification as a Cochrane review.
SR authors’ concluding statements regarding the outcome effects and their findings, along with the strength of evidence, were extracted verbatim and listed in quotations.
The data extraction form included a total of 237 candidate variables. These variables represented all outcome variables of interest to the investigators of this review and included potential moderating or confounding variables.
Efforts were made to minimize the risk of incorrect data entry between the two independent reviewers of this SR. This required the development of an electronic data entry form using Microsoft Access.28 The data extraction tool was reliability-tested between the two reviewers and used all included studies. All the variable results were used for reliability testing of the data extraction form, except the outcome variables. The data extraction was expected to demonstrate a kappa statistic that minimally reflected a 0.74 or greater agreement before its use on the studies selected for inclusion.15 Any discrepancies between reviewers were reconciled.
An MA was conducted on similar studies with shared outcome effects; however, the RCTs retrieved did not report on all a priori predefined outcomes of interest to the reviewers of this SR/MA.
The specific methods used on how missing data were handled in each study (intention-to-treat (ITT), Bayesian methods, imputation methods, last observation carried forward (LOCF)) were reported in this SR. ITT analysis was evaluated by the reviewers to determine if the specific method used by the authors was reported and whether this was described clearly in the respective methods section.29
Reliability testing of data extraction between reviewers
Dichotomous variables were compared between the researchers using cross-tabulations. Per cent agreement and kappa statistics were calculated. The kappa statistics representing each of the categorical variables were averaged, with a mean kappa of 0.33, which indicates poor agreement between the coders of the data extraction coding form used.
Continuous variables were compared between the researchers using correlation matrices. Pearson’s correlations were obtained for each respective continuous variable and then averaged. The mean Pearson’s correlation was 0.75, indicating good researcher agreement. This contrasted with the kappa result for categorical data.
This difference likely reflects the inherent subjectivity in the dichotomous variables, which included quality assessment judgments. Continuous variables represent more objective quantitative measures, for example, hemoglobin A1C. Another possibility may have been differences in background knowledge between the reviewers. The third source of discordant data extraction was the data extraction tool itself, which included questions that may have been ambiguous between the reviewers. Regardless of the source of discordant data extraction, all disagreements were reconciled in a series of meetings. A general reliability calculator was used for this process.30
Assessment of risk of bias in the included studies
The assessment of risk of bias in each of the studies included in this SR relied on the following considerations used in the Cochrane Collaboration’s risk of bias table format for an SR15 21:
Allocation sequence generation (randomization status and method of randomization reported).
Allocation concealment (concealment of the order for random allocation from the investigators assigning individuals to treatment groups).31
Blinding (blinding status of participants, investigators, and outcome assessors).31
Incomplete outcome data addressed (procedures used to address missing data).
Free of selective reporting (selective reporting of outcomes already prespecified in the protocol or in the methods section).
Free of other bias (threats to validity related to the specifics of the study design used, including early stopping of a study, imbalance at baseline between the comparison groups such as disease severity, high suspicion of confounding/effect modification, funding source, and unpublished abstract).
These risk of bias considerations were extracted and entered in the data coding form.15
Funnel plot assessment was conducted on the studies included in this review, along with Begg’s and Egger’s statistical tests, to evaluate for reporting bias.15 17
Measures of treatment effect
The effect measures included dichotomous events/non-events data. These effect measures were used to generate the RR for dichotomous data. MD or SMD was used for continuous data. MD was favored for reporting in this SR provided the scale and the measurement were comparable, as it is intuitively better understood by the reader compared with SMD.
Dichotomous events/non-events data that were used to calculate the effect estimate RR included the following:
Proportion with amputations.
Proportion with complicating infections.
Proportion with DFU completely healed.
Proportion with DFU recurrence.
Continuous data MD and SMD included the following:
QoL index.
Time to complete healing.
Treatment cost.
Statistical analysis
Effect size (ES) estimates for the association of each independent variable with each of the outcomes were extracted using an ES extraction program.30
Dichotomous outcomes were reported in this SR for each of the included studies from events/non-events data for both the intervention and the control groups. The results that were extracted from the dichotomous events/non-events data were subsequently converted into respective proportions and the effects transformed into RR ratios and risk difference (RD). The numbers needed to treat for an additional beneficial or harmful outcome were calculated.18
Time to complete healing and the cost of care were reported as continuous ES measures using MD along with the respective SD for both the intervention and the control group.
QoL was reported using subjective questionnaires on an ordinal scale. These scales were not standardized across studies using similar debridement interventions. ES were converted to MD if comparable scales were used or to SMD (d) if the scales differed.
Procedures were used to compare the effects of the interventions regardless of the outcome metrics used. These conversions/transformations were contingent on the same intervention being used in at least two studies.
ES transformation of the statistical information reported into uniform ES estimates for analysis (RR, MD, SMD) was conducted using HLS-Meta V.0.9 software.30 32
Unit of analysis issues
There were studies reporting simultaneous treatment of DFU on multiple sites for the same patient and the reviewers used the outcome assessment on the most severe DFU present.
Few studies reported multiple time points or observations for an outcome (repeated measurements). Most studies reported postdesign final observations (PDFO). The studies reporting multiple time points were reported as PDFO for both the intervention and the control group in this SR. This review prioritized the last reported final outcome responses to debridement interventions as these were considered a better long-term indicator of outcome success/failure.
Study treatment of missing data
The method used by each study’s authors to address missing data was scrutinized in this SR.29 If studies with continuous outcome measures reported ES but did not report the associated SD, then the respective continuous study outcome was not used in the MA component of this SR. In studies reporting dichotomous outcomes, if the actual raw number of events/non-events was not reported and could not be extracted or calculated from a respective study, then the outcome was not included in the MA component of this SR.
Assessment of reporting biases
Funnel plot asymmetries of ES were used to assess for reporting biases associated with a propensity to determine if positive studies were reported disproportionately as compared with negative studies.14 15
Tests used to assess publication bias in this MA included the Begg’s technique (non-parametric rank correlation test).33 Bias could not be ruled out if the test result was non-significant and this was used complementary to the funnel plot.33 An alternative graphical test, the Egger’s technique, was used to detect bias in this SR/MA.34 The Egger’s technique uses ES and precision, as complementary to the Begg’s technique, which relies on ranks.33 34
Data synthesis
A random-effects model (REM) was used in this analysis due to the difficulty in assuming that an intervention has a singular fixed effect in complex biological systems treated with healthcare interventions.14 15 35 Treatment effects were expected to vary widely in practice, consistent with competing standards of care among the healthcare community, including patients’ biological and genetic variability. This analysis accounted for both conditional and random variability.14–16 36
Sensitivity analysis
Efforts were made by the investigators of this SR/MA to determine whether decisions made during the review process were robust, such as the inclusion/exclusion of studies in the MA with missing or insufficient data, including those available only as abstracts. Studies restricted to unpublished abstracts37 38 were removed and an MA was then conducted separately to determine if the summary effect estimates were robust. This was conducted to determine if pooled ES estimates were disproportionately influenced by the inclusion of two studies available only as abstracts.
The effect estimates using an REM were compared with a fixed-effects model (FEM) to determine if findings were robust despite the type of model used in the analysis.
Results
Description of the included studies
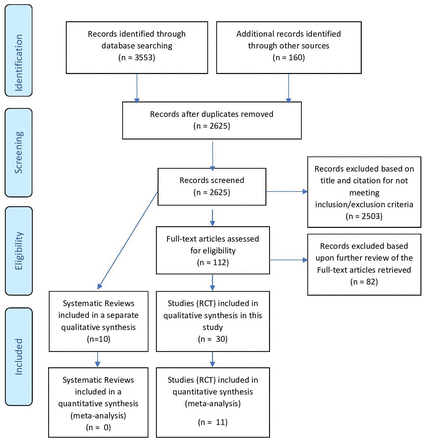
The study search yielded 10 SRs and 30 RCTs that met the inclusion/exclusion criteria for this study (see figure 1).
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial.
SRs retrieved
The prior SRs/MAs varied in the type of clinical trials included. Out of the 10 SRs, 4 included non-randomized studies, and 6 out of these 10 SRs retrieved synthesized the results into an MA (table 1).
The 10 SRs included 4–10 studies. Six of the SRs were restricted to randomized studies, whereas four SRs included both randomized and non-randomized studies. The total sample size of the studies included in the captured SRs ranged from 149 to 575 participants. The follow-up period of the studies included in the SRs ranged from 10 days to 6 months. The study period ranged from 1992 to 2007 and the publication years ranged from 1999 to 2013.
The types of wounds in the studies included in the SRs retrieved varied. Out of the 10 studies, 2 included venous ulcers in addition to diabetic foot ulcers and 1 included ischemic ulcers in patients without diabetes.
The number of comparisons ranged from one to four methods of debridement in the studies that were analyzed in the 10 SRs retrieved (table 1). The types of debridement included sharp/surgical, autolytic (hydrogel, foam, alginates, hydrocolloids, semipermeable polymeric membranes, silver-containing), larva or maggot debridement, and hydrotherapy.
The outcome measures of interest included amputation frequency, infections rates, complete healing rates, time to complete healing, wound size reduction, health-related QoL measures, wound recurrence, and adverse events.
Of the 10 SRs, 5 were Cochrane reviews. The 10 SRs varied in their findings on the quality of evidence, ranging from low evidence to no evidence that any form of debridement, other than autolytic debridement, was beneficial. The conclusions of the authors of two studies suggested low to moderate evidence that forms of autolytic debridement were beneficial.
MA was not conducted to synthesize findings in 4 out of the 10 SRs retrieved (table 1). One MA pooled both randomized and non-randomized studies. The Cochrane reviews included SRs that were updates of previous Cochrane SRs.
SR and MA
Our search retrieved 30 RCTs. These included all RCTs captured and retrieved in the 10 prior SRs for investigation in this review (online supplemental appendix table 2).
The characteristics of the 30 included studies were summarized and assessed for quality (online supplemental appendix file 3A). The Cochrane Risk of Bias tables assessing risk summarize the 30 included studies (figure 2). Collectively, the retrieved studies in this SR included 2564 participants (online supplemental appendix table 3).
(A) Methodological quality graph: review authors’ judgments about each methodological quality item are presented as percentage across all included studies. (B) Methodological quality summary: reviews authors’ judgments about each methodological risk of bias item for each included study are presented as a percentage across all included studies.
Inclusion/exclusion criteria
There was significant variability in the inclusion and exclusion criteria across studies (online supplemental appendix table 2).
Study duration
Study duration ranged from 10 days to 24 weeks and the follow-up period included the years 1992–2012 (table 2).
Descriptive summary of the 30 included studies used in this systematic review and meta-analysis
Study setting
The study settings of the included studies were variable (table 2). For studies listed by country, see online supplemental appendix table 2.
Sample size
Sample sizes ranged from 18 to 619 participants in the individual studies included (table 2 and online supplemental appendix table 4).
Age
Ages ranged from 52.1 to 69.3 years.
Gender
Gender was not evenly distributed, with male participants ranging from 12 to 240 and female participants ranging from 1 to 88. Most participants were male, with two studies reporting one or no female participant (table 2 and online supplemental appendix table 4).
Ethnicity
Ethnicity and body mass index were reported in 4 of 30 and 5 of 30 studies, respectively (table 2).
DFU-specific characteristics of the included studies comprised grading, surface area, and depth. The Wagner grading of DFU and the University of Texas classification system were used in 13 studies (online supplemental appendix tables 5 and 6).
DFU severity
DFUs were classified and included severity reports up to Wagner grade 4 or Texas grade 3. Seventeen studies did not specify a classification and referred to the DFU as partial or full thickness. The initial size of the DFU was specified in 20 of the 30 studies using wound surface area. The size of the DFU was reported for both the intervention and the control group in each study. The depth of the DFU was specified in 5 of the 30 studies. Out of the 30 studies, 14 reported on the duration of the DFU, ranging from 1 week to 15.8 years (SD=10.7). The initial wound stage varied among the 30 studies (online supplemental appendix table 7).
Prognostic risk factors in the included studies
Studies that reported comorbidities potentially complicating healing of DFU included the mean duration of diabetes, which ranged from 13 (SD=10.6) years to 20.5 (SD=13.5) years. Of the 30 studies, infection status of participants was reported in 11, offloading status was reported in 9, and immunosuppression status was reported in 1. Two studies included albumin as a baseline nutritional status indicator. Smoking status was reported in five studies, whereas baseline venous insufficiency status was not reported. Industry support status was reported in 13 of the 30 included studies (table 2 and online supplemental appendix tables 8 and 9).
Results of the search
Three separate searches collectively retrieved a total of 3553 citations (see PRISMA flow diagram in figure 1).
Excluded studies
On review by two independent reviewers, 2503 citations were rejected for not meeting the inclusion/exclusion criteria for this SR based on title citation and/or abstract.
A total of 82 out of 112 studies that could not be excluded based on title citation and/or abstract alone were excluded from this SR upon full-text article retrieval and review. The reasons cited for exclusion of the 82 studies retrieved in full-text for further review are detailed in online supplemental appendix file 3B and included the following:
Not randomized: 30 of 82 studies.
Intervention not classifiable as a recognized primary form of debridement: 30 of 82 studies.
Other debridement interventions besides the comparison interventions were applied to both treatment arms: 12 of 82 studies.
Other reasons specified: 10 of 82 studies.
Risk of bias in the included studies
The method of randomization was unspecified, with the exception of five studies.39–43 The method of sequence generation included simple randomization (1 of 30) or was unspecified in 25 of the 30 studies. Computer random sequence generation was used in 4 of the 30 studies. Allocation concealment was assessed as a form of selection bias and 5 of the 30 studies reported whether allocation concealment was used40–44 (figure 1 and online supplemental appendix file 3A).
Blinding of outcome assessors was reported in seven studies.37 41 45–49 Three studies36 50–52 reported double blinding, including outcome assessors and in the delivery of the intervention. Double blinding was not uniformly reported or clearly defined. Blinding was either not conducted or unclear in the remaining studies. This may have been attributed to the nature of the interventions used (figure 1 and online supplemental appendix file 3A).
Incomplete outcome data were assessed as a form of attrition bias. One study53 reported that there were no dropouts in the study. Another study54 reported withdrawals; however, the reasons were unclear and the method to address withdrawals was not specified.
Eight studies38 48–50 55–58 did not report participant withdrawals nor was the method to address missing data specified. Eight studies46 47 59–64 reported participant withdrawals or dropouts and cited the reasons; however, the methods to address missing data were unspecified.
Two studies52 65 reported that ITT was conducted but did not specify the method used. Three studies41–43 reported using ITT and specified that the method used was LOCF.
Selective outcome reporting was assessed as a form of reporting bias. No prestudy protocols were available for any of the RCTs retrieved. Assessment of selective reporting was based on discordance between prespecified outcomes reported in the methods section, but not appearing in the results sections of the studies. Of the 30 studies, 14 were at low risk of selective reporting, and approximately 11 studies were at high risk of selective reporting based on discordance in outcomes reporting between the methods and the results sections of the studies.
Of the 30 studies, 17 were at high risk of other potential sources of bias. In 13 studies, it was unclear whether other potential sources of bias were due to insufficient information. Information regarding sources of confounding was not consistently or uniformly reported across RCTs retrieved, making it unclear whether disease severity was adequately balanced between treatment arms.
Randomization in studies to balance unknown/uncontrolled confounders in both treatment arms is tabulated66 (see figure 1 and online supplemental appendix file 3A).
Studies had a broad range of follow-up periods, from 10 days to 24 weeks (table 2 and online supplemental appendix file 3A).
Of the 30 studies, 13 received private sources of financial support, 2 reported no financial support, and in 15 studies this was unreported (table 3 and online supplemental appendix table 9).
Summary of results, overall effect sizes, and heterogeneity
Effects of interventions
Out of the 30 included studies, 22 collectively included 19 distinct comparisons and reported on a minimum of one of the seven prespecified outcomes of interest for this SR (table 3 and online supplemental appendix table 10).
Of the 30 studies, 8 studies40 42 43 45 48 49 57 67 did not report on any of the prespecified outcomes of interest despite meeting the inclusion criteria for this review (table 3 and online supplemental appendix table 10).
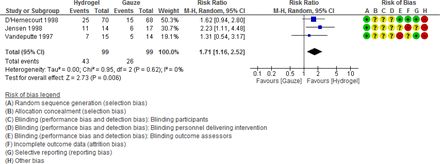
The following were four pooled comparisons with two or more studies: comparison 6 (hydrogel vs gauze),52 63 64 comparison 10 (foam vs wet to dry),56 59 comparison 13 (foam vs wet to dry),41 47 and comparison 19 (hydrofiber vs gauze) (table 3 and online supplemental appendix table 10). Hydrogel demonstrated an increase in the number of DFUs healed as compared with control when three studies were pooled: RR 1.71 (1.16 to 2.52), or a 71% increase in DFUs completely healed (figure 3).
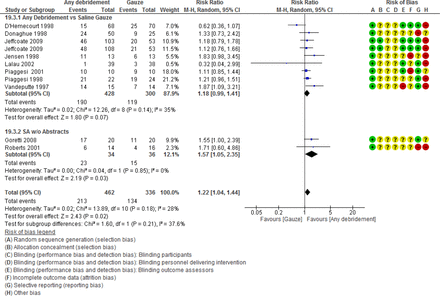
Any form of debridement was associated with increased number of DFUs healed as compared with control in the 11 studies that were pooled: RR 1.22 (1.04 to 1.44) (figure 4).
Forest plot for comparison 19.3: any debridement compared with saline gauze control; outcome: number of diabetic foot ulceration completely healed. Data adapted from D’Hemecourt et al,52 Donaghue et al,65 Jeffcoate et al,41 Jensen et al,63 Lalau et al,46 Vandeputte and Gryson,64 and Piaggesi et al.47 50M-H = Mantel-Haenszel, SA = Sensitivity analysis.
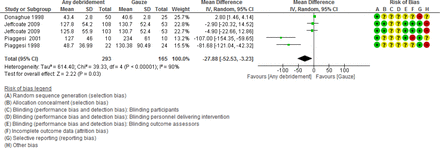
Time to complete healing was observed to decrease with any form of debridement as compared with control by 27.88 days (−52.53 to –3.23) in the four studies pooled (figure 5).
The other outcomes of interest were either not reported at all for hydrogel or for ‘any debridement’ as compared with control, did not demonstrate any statistically significant findings, or were not reported in at least two or more studies. No other statistically significant effects were observed for the other comparisons analyzed (online supplemental appendix figures 1–11).
Supplemental material
Sensitivity analysis (SA)
Sensitivity analysis was conducted by removing the two studies that were only available as abstracts in comparison 19 (any debridement as compared with control).37 38 This was done to determine if the results were robust despite their exclusion from the analysis. Prior to excluding the studies from the analysis, there was a statistically significant increase in the proportion of DFUs healed using an REM, but the ES diminished and there was no statistically significant difference when the two studies available only as abstracts were excluded (figure 4).
The FEM demonstrated no statistically significant benefit irrespective of whether the abstracts were included or not for any debridement as compared with control. Table 3 contrasts the FEM versus the REM estimates. The findings were robust irrespective of the model used. The exception was in the comparison of foam versus wet to dry debridement for the outcome proportion of DFUs healed, where the FEM demonstrated a statistically significant increase in the proportion of DFUs healed, while the REM did not. Any debridement as compared with the control condition demonstrated the mean time to complete healing to be longer in the intervention group using the FEM but shorter in duration using the REM.
Publication bias investigation
Ten studies used in the comparison of any debridement as compared with gauze for the outcome proportion of DFUs healed were plotted in a funnel plot to investigate publication bias. The funnel plot suggested slight asymmetry favoring disproportionately positive studies to the right side of the graph, including the smaller studies which suggested publication bias (figure 4 and online supplemental appendix figure 12).
However, Begg’s and Egger’s statistical tests did not detect any significant evidence of publication bias (online supplemental appendix table 11).
Meta-regression analysis
A meta-regression (MR) analysis was conducted on suspected moderating covariates that may result in effect modification/interaction or confounding. This could magnify or diminish intervention effects. MR was conducted to understand any unexplained heterogeneity. This SR/MA demonstrated relatively low heterogeneity as evidenced by the statistical tests reported in table 3.
The tests for homogeneity including τ2, χ2, and I2 demonstrated large and significant heterogeneity in one outcome (time to complete healing) for both the hydrofiber and ‘any debridement’ interventions as compared with control. Moderate heterogeneity, although not statistically significant, was suggested in the outcome proportion of infections for the hydrogel intervention as compared with control. The outcomes proportion of infections, proportion of DFUs healed, and proportion of recurrence demonstrated moderate heterogeneity for the ‘any debridement’ intervention compared with control, although this was not found to be statistically significant.
The optimal number studies for each moderator/covariate investigated is 10. That is a ratio of 10 studies:1 moderator/covariate investigated for each of the outcomes of interest. This condition limited the MR analysis to on moderator at a time for the the comparison ‘any debridement’ as compared with control.
The moderators investigated included the sample’s risk-specific characteristics (age, PAD, duration of diabetes, and gender) and the study-specific characteristics (data collection year and study duration of follow-up).
Two outcomes satisfied the minimal 10 studies per covariate requirement: proportion of infections and proportion of DFUs healed. Each of these outcomes included 10 studies, although as previously mentioned not all studies reported on every moderator of interest (online supplemental appendix tables 12-14).
Assessment of heterogeneity
Methods for identifying statistical heterogeneity included visual graphical analysis of forest plots and use of Q-statistic, τ2, χ2 test, and I2 test statistics. These statistical techniques were used to both detect the presence and the magnitude of heterogeneity.15 68 69
Residual unexplained heterogeneity as a function of a single outlier effect or exaggerated idiosyncratic effects among outlying responders was evaluated by reviewing the study reports. The effects of outliers were either not reported or the threshold for defining outliers was not standardized across studies included in this SR.
This SR relied on summary statistics, which poses greater challenges in determining the reasons for within-study variance. The risk of bias tables and the tables on the characteristics of included studies served as the basis for clinical and methodological considerations.14–16
The studies varied in type of debridement comparisons and characteristics, including design-specific and sample-specific characteristics.
Covariates of interest to the researchers of this SR were not uniformly reported across RCTs, precluding comprehensive MR analysis. This segment of the analysis includes modeling each of the moderators specified to determine if any effect on the between-study variance exists irrespective of the lack of significant heterogeneity. The analysis included a series of models that used one covariate per model. The use of more than one moderator, in a multivariate approach to modeling, was precluded by the limitation in the information reported and the finite number of studies available.
Weighted mean ES by the inverse of the variance in each study was calculated across all studies under the random-effects assumptions for use in the MR analysis. To assess whether the moderators explain the heterogeneity in the ES, a moderator analysis using weighted mixed-effects models with maximum likelihood estimation of the random-effects weights was conducted. The moderator analysis was conducted using Comprehensive Meta-Analysis.70
The moderator analysis displayed in online supplemental appendix table 12 demonstrates no statistically significant effect on the outcomes proportion of infections and proportion of DFUs healed using all the moderators discussed. The comparison of the new model with the null model for τ2, I2, Q, and R2 does not suggest a significant effect on heterogeneity by including any of these candidate moderators.
Discussion
This SR/MA/MR includes a comprehensive search of the literature using established standards. Our group appraised all available evidence strictly from human experimental studies (RCTs) on debridement of DFU. The evidence included clinical and public health outcomes of interest using different forms of debridement as the treatment intervention for DFU. Thirty RCTs, including 19 debridement comparisons and 7 prespecified outcomes of interest, were investigated. The comparisons were based on data extracted from individual studies to conduct qualitative and quantitative SR/MA. Studies involving four of the separate comparisons studied were synthesized and pooled into an MA. There was no statistically significant beneficial effect in preventing amputations or reducing infection rates in any of the comparisons analyzed with MA (table 3).
QoL was reported in three studies and there was no significant difference found between the form of debridement as compared with the control condition using the Short_Form - 36 (SF-36) QoL questionnaire41 50 52 (online supplemental appendix figure 15).
Studies that reported on DFUs healed as the outcome failed to demonstrate a statistically significant difference in healing between specific forms of debridement as compared with control, with two exceptions: hydrogel (autolytic debridement) and ‘any’ form of debridement. Both analyses demonstrate a statistically significant increase specific to the outcome proportion of DFUs completely healing (table 2 and figures 3 and 4).
When the two studies only available as abstracts were excluded in a sensitivity analysis, there was no significant difference in complete healing with any debridement as compared with control (table 3 and figure 5).37 38
Any debridement versus control demonstrates a significant reduction of approximately 28 days to heal using the REM, whereas a 2.5-day decrease was observed when using the FEM (see table 3 and figure 5). The REM was specified a priori since this was considered to be appropriate in a study of this nature and the complex analysis involved. The REM provides a result that is considered the average intervention effect from a distribution of effect estimates across studies, and that the variation is due to genuine variation in effects across all studies that is not solely due to random error or chance,71 whereas the FEM assumes that the intervention is the same across all studies and any variation is due to random error or chance.71
The REM was prespecified for this SR, although findings were robust irrespective of the model (REM vs FEM) used, with three exceptions. The FEM demonstrated a significant beneficial effect on the proportion of DFUs healed with foam as compared with the wet to dry debridement intervention; however, this was not apparent when using the REM (table 3 and online supplemental appendix figure 8).
The any debridement group compared with control found a significant beneficial effect in proportion of DFUs healed using both the REM and the FEM. However, when studies only available as abstracts were excluded from the analysis, both models failed to demonstrate a statistically significant difference in the outcome proportion of DFUs healed and the ES was reduced (see table 3 and figure 4).
The MA using foam compared with wet to dry debridement demonstrated a significant beneficial difference in the FEM but not the REM for the outcome proportion of DFUs healed (table 3 and online supplemental appendix figure 8).
In the pooled studies reporting recurrence rates, no significant beneficial difference was observed between the competing forms of debridement or ‘any debridement’ as compared with the control condition.
Studies retrieved in this SR included trials using smaller sample sizes, which may have been statistically underpowered. This creates difficulty in detecting statistical significance if the debridement intervention is associated with small treatment effects.
An MR analysis found that none of the candidate moderators was predictive as a univariate model for any of the respective outcomes of interest. There was no significant study heterogeneity explained with any of the univariate models evaluated in this MR analysis (online supplemental appendix tables 12-14).
Variability in study reporting limited the choices of moderators available for analysis in the MR component of this SR to a smaller subsample of studies retrieved. The effects of these moderators on the outcomes of interest will makee further investigation possible with improvements in standardization of reporting across RCTs. This would increase the sample sizes available for MR analysis and improve detection of significant effect modification/interactions.
Limitations
Overall completeness and applicability of evidence
QoL and cost of treatment were not well defined. For example, an acceptable robust standardized QoL measure, such as SF-36 or a similar measurement tool, was universally underutilized in the studies retrieved for analysis. QoL and treatment cost are fundamental considerations when comparing the various debridement methods using standardized reporting guidelines. Critical outcomes of interest between studies, including amputation and infection frequency, were variably reported.
Indirect evidence applicable to the primary outcomes of interest is provided by prespecified secondary outcomes, including recurrence rates, complete healing, and time to complete healing.72 These outcomes were also variably reported throughout the 30 included studies, making meaningful comprehensive data synthesis a challenge.
Quality of evidence
All 30 studies used in this SR can be classified to be of unclear or high risk of bias (figure 2 and online supplemental appendix file 3A). Allocation concealment was unclear in greater than 75% of the studies. Due to the nature of the interventions, blinding may have been challenging to the participants and to the personnel delivering the intervention. Blinding was often absent or incompletely reported. Blinding of outcome assessors was unclear in approximately 70% of the studies.41 45–49 51 52
Incomplete outcome data reporting was unclear or high risk in over 75% of the included studies. Selective reporting of outcomes was unclear or high risk in 50% of the included studies. Other bias was either unclear or high risk in the included studies.
The studies did not follow established reporting practice such as the Consolidated Standards of Reporting Trials Consoluidated Standards of Reporting Trials (CONSORT) statement.73 Major considerations of these guidelines include appropriate random sequence generation and allocation concealment. Thirty studies reported randomization; however, only five of the studies reported the specific method used.39–43 Allocation concealment was reported in 5 of the 30 studies.40–44
The CONSORT guideline standardizes reporting guidelines and was developed for research investigators to better define and reduce variability in reporting in the medical and public health literature. Standardization would make it possible to better synthesize the evidence in SRs and MAs.
The methods to address incomplete outcome data were not reported in most studies and four studies reported ITT analysis using LOCF.41–43 52 Fundamentally, better efforts should be made in the planning and conduct of the study to limit missing data. ITT methods used were not uniform and varied across the included studies.
Selective reporting of outcomes was difficult to determine as all studies did not provide a prestudy protocol. The investigators of this SR/MA made efforts to determine whether all outcomes defined and reported in the methods sections were subsequently reported in the results sections of the included studies. The approach used in this SR was a surrogate means of detecting selective reporting bias in the studies retrieved. Many studies were characterized as unclear or high risk of bias with respect to selective reporting of outcomes. Prespecification of all outcomes of interest reported in the methods section should summarily be reported in the results section of the RCT. Any deviation by the investigators of the RCTs should be clearly explained.
The included studies under-reported disease severity and used unclear method(s) of measurement; for example, it was unclear how PAD was assessed as this may confound the results if competing interventions such as revascularization were used concurrently.
Of the 30 studies, 13 were industry-supported, but the studies did not demonstrate significant evidence of publication bias in this SR. Assessment of publication bias was limited to ‘any debridement’ as compared with control for the outcome number of DFUs healed.
Potential biases in the review process
The investigators of this SR/MA/MR made efforts to include studies not published in English. Studies were translated using Google Translate and outside translators were used when Google Translate information was incomplete.74 The potential for bias exists when translating studies. However, the results from translated studies were concordant with other published studies used in this review.
Efforts to contact the authors of the two studies available only as abstracts were unsuccessful.
Studies reported paired intervention treatment arms with another form of debridement, for example, using sharp debridement concurrently. This occurred when sharp debridement was not one of the primary treatment arms studied. These studies were excluded unless regular use of sharp debridement in both intervention treatment arms was reported. However, this made it difficult to determine inherent efficacy and exclude confounding of the primary debridement methods by use of concurrent sharp debridement in both treatment arms.
Studies with short-term follow-up periods were compared with studies using longer follow-up periods, ranging from 10 days to 24 weeks, which could have introduced bias. Length of study was not used as inclusion/exclusion criteria in this SR/MA in an effort to avoid missing pertinent studies during the search process.
RCTs published since the conduct of this SR
An RCT using ultrasound debridement was not available at the time of this review but was underway and published in 2019.75 An additional RCT on maggot debridement therapy was also concluded in 2019.76 The authors of these RCTs reported favorable outcomes on the role of these debridement interventions in DFU. These RCTs will be reviewed and may meet eligibility criteria in a future update of this SR. If additional RCTs on these interventions are conducted, it may also be possible to synthesize the evidence and generate better inference from future updated MAs.
Three studies were designated ‘studies awaiting classification’. Determination of inclusion/exclusion criteria could not be made until additional information becomes available.
Agreements and disagreements with other SRs
A summary table compares and contrasts the 10 prior SRs retrieved to the findings of this SR (table 4).
Summary of a comparison of the systematic reviews preceding this current systematic review
The SRs included 4–10 studies. The SRs all included randomized studies, and 3 of 10 SRs included non-randomized studies.77–79 The number of participants ranged from 149 to 575 participants. The follow-up period in the retrieved SRs ranged from 10 days to 24 weeks. The study period included in the SRs ranged from 1989 to 2007.
The 10 SRs retrieved included 5 SRs that used a standardized approach to summarize their findings. These five SRs were Cochrane reviews that used the GRADE approach.80 The 10 SRs pooled 2–6 studies in their respective analyses. The retrieved SRs’ findings collectively concluded no, low, or weak evidence that one form of debridement was superior to another form of debridement or superior to the control condition.20 None of the 10 SRs used MR or conducted any type of moderator analysis.
The summary of our review’s findings was based on the GRADE approach.80 Many of our conclusions regarding the direction of future trials were similar to those of the preceding SRs (online supplemental appendix tables 15-18). These include findings that demonstrated low evidence that any form of debridement type is more effective than other forms of debridement in healing DFUs. There was low evidence for hydrogel and for any debridement when compared with control, and these findings remain unclear due to risk of bias (online supplemental appendix tables 15-18). The SRs that included non-randomized studies with RCTs made comparable conclusions to systematic reviews restricted to randomized studies.
The findings in the 10 included SRs were consistent in that they reported weak or poor evidence to conclude that one form of debridement was superior to alternate forms of debridement or to the control condition/standard treatment, including wound care and saline/antiseptic dressing. The SRs uniformly reported significant sources of bias in the respective RCTs retrieved for analysis.
Due to the variation in research methods and study types included in the 10 SRs retrieved, our research team made the decision not to attempt synthesis of the findings by performing a MA on the retrieved SRs. Instead, we reported the authors’ results of the 10 SRs qualitatively, along with their respective and consistent conclusions. Drawing statistical inference by combining data extracted from SRs that have variable designs includes the following challenges: SRs that combined both randomized and non-randomized trials, variation in the RCTs retrieved, inconsistency of outcome findings reported, and variation in the wound types studied. This made further study with quantitative MA impractical. There was also variation in the type and quality of quantitative information reported among the 10 included SRs. The form of debridement most frequently used as a control condition or standard treatment condition or comparator was autolytic debridement, specifically using moistened gauze dressings with either saline or an antiseptic such as iodine.
Conclusions reported in the prior SRs retrieved with regard to the direction of future trials included the need for larger sample sizes and standardized reporting from the authors of the clinical trials. The findings in these SRs were consistent in that they found weak evidence that any debridement or debridement dressing type was more effective than other forms of debridement in healing diabetic foot ulcers. The authors reported increased risk of bias in the included studies used in the 10 SRs retrieved for this analysis.
GRADE evaluations were used on the four MAs with significant findings conducted in this SR . These included hydrogel compared with control (online supplemental appendix table 15), foam dressing compared with wet to dry saline dressings (online supplemental appendix table 16), hydrofiber compared with control (online supplemental appendix table 17), and any debridement compared with control (online supplemental appendix table 18).
The GRADE approach was used to objectively evaluate the evidence and concluded the following80: the quality of evidence is low to very low for the comparisons studied and was found to be weak based on the considerations used in the GRADE approach.80
These findings do not support the endorsement of any single form collectively as superior to any other form of debridement in the treatment of DFU, nor do the findings support the use of any form of debridement over the control for comparison.81 This SR may be considered a non-inferiority study. The CIs for the point estimates were large, frequently including thresholds of equivalence, which was consistent in the analyses conducted.
Conclusion
Patients, healthcare providers, policy makers, and all other stakeholders are cautious in altering clinical practice on the basis of findings derived from small trials of unclear or high risk of bias, including non-randomized studies or weakly designed randomized studies.
Stakeholders are careful not to extrapolate findings from DFUs to other wound types, although diabetic wounds are considered among the most recalcitrant of wounds. Findings related to these resistant wound types may be applicable to researchers studying wounds other than DFUs. The findings in the review of the literature on SRs conducted on similar research questions supported the need to design and conduct a comprehensive SR that uses all available human experimental evidence from all RCTs, including those RCTs in the 10 SRs reported previously. This may assist diverse stakeholders in making important clinical, public health, and policy decisions.
Implications for practice
This comprehensive SR of all available human experimental evidence in RCTs supports the conclusion that no specific debridement method used in DFU appears to increase healing rates as compared with other debridement methods. This includes the control using moistened gauze and standard practice. The included studies evaluated debridement interventions on participants with a wide variation in grade/stage of DFUs. It was unclear whether disease severity and comorbidities in the RCTs were balanced between interventional treatment arms. This was apparent in smaller studies despite the use of randomization to balance for confounding.
Practitioners may choose to consider other characteristics such as individualization of therapy, patient tolerability, indications/contraindications, and cost when choosing between alternative methods of debridement. These challenges can be appreciated since uncertainty exists around this treatment decision based on the quality of data available to inform clinical decision making.
Implications for research
Currently, inadequate evidence exists to conclude there is any difference, advantage, or benefit between various competing forms of debridement as compared with the control condition, a form of autolytic debridement using moistened gauze as standard care.
It is important that future studies include standardized outcome reporting, including QoL indicators, and cost-effectiveness analyses, which were underutilized in the studies retrieved. The need for less variability and more uniform reporting of specific outcomes may have direct implications on clinical decision making and public health, including the outcomes frequency of amputation, complicating infections, and QoL. Future standardized research methodology will be of value to patients and all stakeholders.
It is important to view the DFU as a determinant or risk factor for adverse outcomes, rather than purely as a disease state. The status of other interventions and standards of care in DFU, including offloading, nutritional services, infection eradication, smoking interventions, and PAD interventions, should be universally reported.
The use of the Wound, Ischemia, and Foot Infection system developed to help stratify amputation risk in patients should be strongly considered.82 Its wider use in clinical practice and studies could provide valuable information on disease severity and amputation risk that would be useful to research investigators.
Studies should be conducted in accordance with standardized uniform practice guidelines for design, conduct, and reporting of RCTs. This would afford researchers the opportunity to design and conduct better quality syntheses of evidence using SRs/MAs, facilitating more meaningful inference from data.
The complications associated with amputations, infections, QoL, and premature mortality disproportionately burden individuals, healthcare resources, the society, the family unit, and workplace/employers, along with government and private services for the disabled. SRs are useful tools in evidence-based medicine. They require summarizing and pooling of high-quality studies to make broader inference and help identify knowledge gaps. They will not only summarize and make broader inference on the state of current evidence but will also help direct future research efforts, as highlighted in this SR.
Ethics statements
Patient consent for publication
Ethics approval
IRB or ethics approval was not required as this study is a systematic review and includes summary statistical data extracted from 30 randomized controlled trials. These data were subsequently synthesized for use in meta-analyses. A research study proposal/protocol was created a priori and reviewed and approved in advance of the research investigation by the oversight committee acknowledged in this manuscript and is archived with the University of Connecticut. This research study protocol/proposal is available upon request. This research investigation originated in part as a doctoral dissertation conducted through the University of Connecticut.
Acknowledgments
We are sincerely grateful for all the guidance, support, mentorship, and feedback from Dr Martin Cherniack, Dr Richard Stevens, Dr Nicholas Warren, Dr Thomas Babor, and Dr Scott Wetstone, University of Connecticut Department of Community Medicine and Department of Allied Health Sciences; and for the expertise, support, and assistance of Sally Bell-Syer, Gill Rizzello, Ruth Foxlee, and Rocio Lopez, Cochrane Review Groups - Wounds; Jill Livingston, reference librarian and search coordinator, University of Connecticut, Storrs, Connecticut; Louis Wiethe and Virginia Carden, Duke University Library, Chapel Hill, North Carolina; Kay Dickerson, Tianjing Li, Marie Diener-West, Andrew Law, Kristina Lindsley, and the entire team at the US Cochrane Center at Johns Hopkins University, Baltimore, Maryland; Dr Reza Shah, Department of Surgery, Robert Wood Johnson University Hospital at Hamilton, Robert Wood Johnson - Barnabas Health System; Eileen Egan and Timothy Wages, Phelps Hospital - Northwell Health hospital administration for the financial support in providing open access to all readers. Our profound gratitude to our wonderful families for their support and patience.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors Journal article supervision: DD, TBH-M, and OO’N had full access to all the data in the study and take responsibility for the supervision of the study including the integrity of the data and the accuracy of the data analysis. The authors were responsible for obtaining IRB approval if required. Concept and design: DD, TBH-M, OO’N. Acquisition and analysis of data: DD, TBH-M, OO’N, NH. Interpretation of data for the work: DD, TBH-M, OO’N. Proposal writing and editing: DD, TBH-M, OO’N. Drafting of the manuscript and editing: DD, TBH-M, OO’N, NH, JM, KI. Critical revision of the manuscript for important intellectual content: DD, TBH-M, OO’N, NH, JM, KI. Administrative, technical, or material support: DD, TBH-M, OO’N, NH, JM, KI. Journal format preparation, submission, and correspondence: DD, TBH-M, OO’N.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.