Introduction
The medical world is evolving and innovating faster than ever. In order to prevent harm, this process of innovation requires regulation, derived from evidence-based principles, rather than uncontrolled experimenting. For drugs and therapeutic biological products, the introduction of new agents is strictly regulated and new drugs can only be widely distributed after going through a four-phase regulatory drug approval process and meeting mandatory requirements.1 The process of surgical innovation, however, is complex and has been unregulated and unstructured in the past. This possibly exposes patients to an unethical higher risk and harm. Multiple factors challenge the feasibility of formal assessment of surgical innovations: the intrinsic complexity of surgical techniques, varying expertise of individual surgeons and the constant ongoing innovation of surgery. Although evidence-based healthcare has been endorsed by the surgical community, there is still a considerable lack of well-designed and properly powered randomized controlled trials (RCT).2
In rectal cancer surgery, a tremendous evolution has taken place from open to minimal invasive techniques, and other techniques are being pioneered continuously, with the aim to improve patient outcomes. Nevertheless, the majority of these newer techniques are a pure abdominal approach which remains technically challenging, particularly for low rectal cancers in a narrow deep pelvis. Transanal total mesorectal excision (TaTME) is the latest surgical technique that has the potential to overcome these drawbacks and has attracted a huge interest worldwide. TaTME is essentially an amalgamation of well-established surgical techniques and principles: TME surgery, as proposed by Heald,3 transabdominal transanal approach (TATA), transanal endoscopic microsurgery (TEM) and transanal minimally invasive surgery (TAMIS).4–6
While innovation is exciting, the question remains if these new approaches truly lead to better oncological outcomes and quality of life for patients. Minimal invasive approaches add complexity to the procedure and require an increased skill set. Moreover, patients might be exposed to uncertainties and harm from new complications, inherent in innovative procedures. This highlights the need for a robust framework to introduce new surgical techniques in a safe way and avoid widespread adoption before high-quality assessment has taken place, in order to avoid harm to the patient and to protect the surgeon.
The IDEAL framework (Idea, Development, Exploration, Assessment, and Long-term study) was proposed by the IDEAL collaboration.2 7 8 Their aim was to establish a rational approach and develop an integrated evaluation pathway for surgical and other complex interventions. The framework is a guidance in how to get from an idea to safe implementation, by thorough assessment of all the stages of development of a new surgical innovation. IDEAL promotes a shift away from the traditional, uncontrolled, retrospective case series that compose most of surgical research, towards planned prospective observational studies leading to high-quality RCTs.
Our aim was to describe how TaTME has developed since its introduction, and how this evolution aligned with the steps and recommendations of the IDEAL framework. This is summarised in figure 1.
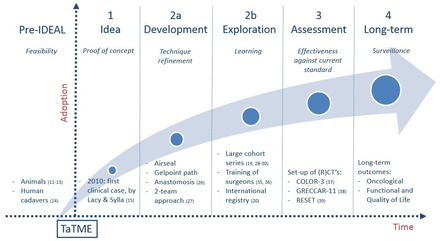
Evolution of transanal total mesorectal excision (TaTME) following the IDEAL framework.
Pre-IDEAL
The pre-IDEAL stage of the framework was recently proposed and added to the initial five-step framework.9 In this phase, the feasibility of a new procedure is tested and further developed on cadavers and animals, before starting clinical application.
The first surgeon to present a series of three human cadavers was Mark Whiteford in 2007,10 who performed sigmoid resections using natural orifice transluminal endoscopic surgery (NOTES) instrumentation. He showed that ‘en bloc’ lymphadenectomy, primary anastomosis and retrieval of an intact specimen could successfully be performed via a transanal approach, without any incisions. The procedural steps he described actually come very close to what we would now recommend as the essential steps for a radical transanal proctectomy.
Accordingly, different aspects of the procedure were further explored and tested in animal models (pigs) and cadavers, trying to establish whether a full NOTES procedure for rectal resection would be possible.11–13
The largest human cadaveric series (n=32) of transanal sigmoid resection via NOTES was conducted between 2008 and 2011, reporting on an intact mesorectum in all the specimens and a trend towards reduction of operative times.14
Stage 1: Idea
Following this extensive preclinical work in the pre-IDEAL stage, focus shifts to the first step of the IDEAL framework. In this phase, the ‘proof of concept’ takes place; it describes the first clinical case of a new procedure, prompted by the need for a new solution for a clinical problem. Only a small group of surgeons, defined as ‘innovators’, start performing the procedure on a few highly selected patients. Small case series are written to report on any favorable outcomes in order to inform other colleagues, yet it may be even more important to also report on recurrent mistakes and errors to avoid their repetition in the future.
For TaTME, the Idea was to apply the advances in transanal surgery (NOTES, TEM, TATA, TAMIS), to further optimize the TME principle, by performing a transanal minimally invasive TME.
In 2010, Sylla and Lacy reported on the first clinical TaTME case.15 A healthy 76-year-old woman, without previous abdominal surgery, diagnosed with a T2N1 rectal carcinoma at 8 cm from the anal verge, was selected to undergo the first TaTME procedure. They performed the procedure through a rigid TEM platform with laparoscopic assistance from above for the initial exploration and sigmoid mobilization. The postoperative course was uneventful and a complete TME specimen was obtained with negative margins. After the success of this initial case, three cases were reported by Lacy et al,16 with negative resection margins, good quality of the specimen and without postoperative complications.
As these early reports suggested safety and even potential benefits, some ‘early adopters’ took up the innovation, and so, around the end of 2012, TaTME moved into the Development phase.
Stage 2A: Development
In this phase, the focus is on technical development and feasibility of the procedure, in an initial small and selected group of patients. The few innovators have developed personal experience, and some early adopters start to join the innovators. Technical modifications of the technique, including the different steps of the procedure as well as changes to the equipment, are common during this phase. A regulatory ethical process is required at this stage to prospectively register all consecutive patients and report on their outcomes.8
A prospective series was presented by the ‘early adopters’ of 20 well-selected patients with rectal cancer, undergoing a transanal NOTES with laparoscopic abdominal assistance between August 2011 and July 2012 at the Hospital Clinic of Barcelona.17 It demonstrated a safe and oncological adequate procedure. Other small series showed the same, encouraging outcomes of TaTME in terms of safety and efficacy.18 19
Collaboration is fundamental for further procedural development, and the few innovators and early adopters of TaTME were aware of this. In March 2013, the first international transabdominal TaTME meeting was held in Houston, involving nine surgeons from the USA and Europe. They shared experiences and pitfalls and discussed how these could be tackled, in a way to find a unique standardization of the technique. Key points of discussion were the difference in appearance of the pelvic anatomy from below, technical challenges related to the purse string and anastomosis, and the need to use a multiport rectal device (GelPOINT path transanal; Applied Medical) rather than the rigid platform, for a safe and atraumatic dissection. Furthermore, collaborators decided to prospectively collect data on their patients for analysis and publication within an international registry.20
A year later, a second International TaTME Summit was held in Paris, after which current status and modifications of the technique were reported in an official consensus statement.21 It outlined three facets of the TaTME procedure: the technique and its indications, training and adoption, data collection and the TaTME registry. Regarding indications, patient selection is difficult in surgery, considering the technique varies among individual surgeons, and patients themselves are varied. The consensus stated TaTME could be used for both benign and malignant procedures where dissection of the distal or mid-rectum is required. TaTME is preferred in males, with low rectal cancers and visceral obesity.
No recommendations were done regarding ethical considerations, although this is crucial at this stage. When developing a new technique, patients are unavoidably exposed to new risks and harm. Well-intentioned surgical experimentation on patients must therefore be regulated and monitored. Bernstein and Bampoe proposed a guideline for determining the need for regulation of novel neurosurgical procedures, and highlighted the need of institutional review boards in this process.22
The colorectal team in Oxford was among the early adopters of this new approach and collaborated with the pioneers to further improve the technique, as there were some concerns. They felt that the new TaTME technique was challenging due to the unfamiliar view from below, and demanded a stable field of dissection to properly view the anatomical landmarks. A new platform (AirSeal; ConMed) for more stable pneumorectum and better smoke evacuation was proposed, resulting in increased visibility with more convenient and precise dissection.23
Another concern was how to define the correct plane, and, once found, to maintain that plane, since again, landmarks are different with the down to up approach. The fear of getting close to the tumor and/or disrupting the mesorectum leads to reverse coning with the risk of going too wide and ending up in the pelvic sidewall, causing bleeding and damaging anatomical structures (nerves, prostate, vagina and urethra are particularly at risk). Bernardi et al demonstrated specific features during dissection to guide surgeons. The ‘triangles’ created using appropriate traction can aid in performing a precise dissection in the correct plane, while features described as ‘O’s can alert surgeons that they are entering a new fascial plane and can avoid incursion into an incorrect plane.24 Especially the risk for urethral injury, which is negligible in conventional abdominal TME, requested new methods for localization of the urethra, as assessed by Atallah et al.25
The formation of a colorectal or coloanal anastomosis is one of the critical steps in TaTME. Compared with standard laparoscopic stapling of the distal rectum, TaTME allows stapling techniques with excellent visualization and avoidance of cross stapling, potentially reducing anastomotic leakages,26 especially valuable in patients with a narrow pelvis. Penna et al proposed four techniques: a hand-sewn technique for tumors reaching the anorectal junction, a stapler device for higher tumors, with the choice for stapler configuration and diameter depending on tumor and patient characteristics and surgeons’ preference.27
As one of the innovators, the group of Lacy at the Hospital Clinic of Barcelona presented the promising outcomes of the standardized and refined technique after performing more than 300 TaTMEs, including the description of two synchronous surgeon working teams, to reduce operative time.28
At this point, the technique was further developed, based on the experiences of the innovators and early adopters and their collaboration. The technical steps of the procedure were roughly defined when a very rapid uptake occurred of many surgeons and centers implementing TaTME. Hence, not all important steps were transferred from the early adopters to the early majority of surgeons, and the transition to the exploration phase occurred at an early stage.
Stage 2B: Exploration
In the exploration phase, attention shifts from developing the technical aspects of the procedure to focusing on correct indications, understanding the potential harms and benefits and planning how an RCT can be initiated. The surgical intervention is more widely used, now performed by the innovators, early adopters and early majority of surgeons. Observational studies should prospectively collect data from multiple centers and surgeons, to ensure measurement and comparison of data. Usually large numbers of patients are needed before an RCT is feasible.8
Several groups published the first large cohort series of TaTME.19 29–31 The largest cohort study was published by Lacy et al, describing the results of 140 patients, reporting a complete resected TME specimen in 97.1% of the cases and low morbidity.
In July 2014, the International TaTME Registry20 was set up, launched by the UK Pelican Cancer Foundation. Ethical approval for the registry was granted by the UK Health Research Authority (REC reference 15/LO/0499, IRAS project ID 156930). It was designed to quickly collect high-quality data of cases performed all over the world, in order to further identify the potential harms and benefits. The registry has a strong international collaboration and reflects a wide community of practicing surgeons with varying levels of experience.
In 2017, Penna et al reported the first 720 cases that were recorded on the registry.32 This was the largest, prospective data series published on TaTME, and an important contribution to the safety assessment of TaTME. Good pathologic outcomes were found: an incomplete specimen in 4.1% and R1 resection (tumor or lymph node ≤1 mm from the resection margin) in 2.7% (16 cases). However, they also reported on some technical difficulties during the procedure, such as an unstable pneumopelvis and smoke-related problems with visualization, leading to visceral injuries, especially five urethral injuries.
Urethral injuries are rarely seen in conventional TME approaches and this type of injury is a specific concern for TaTME. An assessment of the outcomes after cadaveric training in North America33 showed a high (trainee-reported) risk of urethral injury after attending a cadaver training course. This suggests that only cadaver workshops are not sufficient to implement this technique safely and highlight the need for a structured training program.
The second international registry paper included 1594 patients and assessed anastomotic failure.34 An overall anastomotic failure rate of 15.7% was found, which included early (7.8%) and delayed leaks (2.0%). The rate of irradical (R1) resections was 3.9% and urethral injuries were rare (0.8%).
Another rare but clinically important risk associated with TaTME is the occurrence of a carbon dioxide embolus. The first combined paper from the International TaTME Registry and US TaTME registry, currently in press, reports on an incidence of 25/6375 (0.4%). They recommend awareness among surgeons and anesthetists, as early recognition and management can limit the clinical impact of such a complication.35
The advantage of an international registry is to assess the therapeutic effectiveness and safety of TaTME, reflecting ‘real world’ practice, with surgeons at different stages in their learning curve. This offers a quick and early assessment of a new surgical procedure.
Another important issue of this phase is training, mentoring and learning curve evaluation, as the procedure is likely to be adopted by an increasing number of surgeons. On 12 October 2015, the first educational consensus workshop for TaTME took place.36 The need for an agreed training curriculum and how this educational program should be structured was discussed. They proposed the formation of the international TaTME educational collaborative group, to develop a TaTME training curriculum. Following their consensus meeting, they agreed on the steps in the process to achieve a safe implementation: providing shared communication platforms among all stakeholders in the field to drive the educational standard for TaTME, agree on the essential elements of an optimal training curriculum and providing guidance on the implementation and assessment of a training curriculum for TaTME.
Recently, a consensus on this structured training curriculum was proposed by Francis et al.37 They recommended that surgeons aiming to learn TaTME should be accredited in laparoscopic colorectal surgery, with prior experience in transanal surgery. The most important aspects of the curriculum were mentorship, multidisciplinary training, online modules and simulated training for purse-string suturing. Entering data into the registry was recommended, as well as a formative assessment to promote learning and competency.
This structured training program was put into practice into the UK, where a pilot national training program for TaTME was launched in September 2017.
However, due to the promising early results, a rapid introduction of TaTME in many centers without surgeons completing a full training program resulted in an early shift to the Assessment phase.
Stage 3: Assessment
At this stage, effectiveness of the new technique against current standards is assessed. A new intervention has shown early promise and is used increasingly by the surgical community; however, the intervention’s relative benefit compared with conventional approaches is still uncertain. Properly conducted RCTs should be the primary choice, as new techniques are prone to overoptimistic assessment by their developers.8 If an RCT is not feasible, which is not exceptional in surgical innovation studies, alternative designs could be used.7
The outcomes of the International TaTME Registry also contributed to the set-up of three RCTs. The aim of these studies is to validate the efficacy and safety of TaTME, with respect to perioperative outcomes, short and long-term complications and oncological and functional results. The COLOR III trial38 and GRECCAR 11 trial39 are international, multicenter, randomized trials comparing TaTME and laparoscopic TME for mid and low rectal carcinomas. The RESET trial40 will be a prospective, observational, case-matched, four-cohort, multicenter trial. It is designed to study all surgical options available for TME in patients with rectal cancer: open laparotomy, laparoscopy, robot-assisted surgery, or transanal surgery. Participating surgeons treat all their eligible patients with rectal cancer with their preferred intervention. This design minimizes the influence of the learning curve effect and surgeons might be more willing to participate in expertise-based trials.
Attention will shift to the next phase, once valid evidence on the intervention’s relative effectiveness is obtained. At present for TaTME, definitive studies are needed. Furthermore, aspects requiring long-term monitoring should be identified in this stage, in order to set up studies related to the last phase of the IDEAL framework.
Stage 4: Long-term studies
The evolution of TaTME has not yet reached stage 4, in which procedures are assessed for long-term outcomes. Meticulous surgery with clear view of the dissection plane and of the neurovascular bundles should theoretically provide better bladder, sexual and bowel function. Currently, it remains uncertain if this is true, and what the oncological implications are from this technique. However, the International TaTME Registry and the aforementioned trials gather information on long-term oncological status and quality of life and these outcomes are soon to be expected.
In order to collect these data, it is preferable registries are being set up from the start of implementing a new technique (stage 1). This is often not feasible, as at that stage it is still unknown if the technique will be adopted and if setting up a registry is worthwhile. As for TaTME, the registry was set up fairly soon after the introduction of TaTME. This was beneficial for a quick and early overview of early outcomes from a large patient cohort (as described in stage 2B), and will provide data on learning curve analysis, mid-term and long-term oncological outcomes and long-term quality of life and functional outcomes in the near future.
When large numbers of patients have a sufficient length of follow-up, an investigation of outcome variations among subgroups is recommendable, to prevent unfair comparison of results between different centers or surgeons with varying patient groups.