Key Points
-
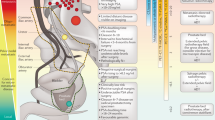
Low-risk prostate cancer, defined as Gleason Score 6 or less with PSA <10 ng/ml, is diagnosed in about half of men undergoing screening
-
About 30% of men diagnosed with low-risk disease harbour high-grade cancer in areas of the prostate detected with difficulty by conventional systematic biopsy: the anterior prostate and anterolateral horn
-
A small percentage of low-grade cancers (1% of patients per year) harbour molecular alterations that result in grade progression, which means that long term follow up is required
-
The primary reason for the US Preventive Services Task Force rejection of PSA screening was a concern about overdiagnosis and overtreatment of clinically insignificant disease
Abstract
Low-risk prostate cancer, defined as Gleason Score 6 or less with PSA <10 ng/ml, is diagnosed in about half of men undergoing screening. Approximately 30% of men diagnosed with low-risk disease harbour high-grade cancer that is unrepresented on the biopsy. Moreover, a small percentage of low-grade cancers have molecular alterations that result in progression to aggressive disease. Favourable-risk prostate cancer should be managed with close follow up. Active surveillance is appropriate for most patients with low-risk disease, and radical treatment should be reserved for cases in which higher-risk disease is identified. In turn, focal therapy aims to preserve tissue and function in men who have been diagnosed with localized disease, and should be offered to men with higher risk disease at baseline, as an alternative to whole-gland radiation or surgery, or when the patient transitions from low-risk to higher-risk disease. The two strategies should be viewed as complementary elements of care that can be applied in a risk-stratified manner. In this Review, we discuss the rationale and current status of active surveillance—which constitutes a standard of care in most evidence-based guidelines—and comment on whether and when focal therapy should complement it in those men wishing to continue a tissue-preserving strategy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Edwards, B. K. et al. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer http://dx.doi.org/10.1002/cncr.28509.
Screening for prostate cancer. A Review of the Evidence for the U.S. Preventive Services Task Force [online].
Sakr, W. A. et al.High grade prostatic intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma between the ages of 20–69: an autopsy study of 249 cases. In Vivo 8, 439–443 (1994).
Zlotta, A. R. et al.Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. J. Natl Cancer Inst. 105, 1050–1058 (2013).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Ahmed, H., Aya, M., Freeman, M. & Emberton, M. Do low-grade and low-volume prostate cancers bear the hallmarks of malignancy? Lancet Oncol. 13, 509–517 (2012).
Ross, A. E. et al. Gene expression pathways of high grade localized prostate cancer. Prostate http://dx.doi.org/10.1002/pros.21373.
Skacel, M. et al. Aneusomy of chromosomes 7, 8, and 17 and amplification of HER-2/neu and epidermal growth factor receptor in Gleason score 7 prostate carcinoma: a differential fluorescent in situ hybridization study of Gleason pattern 3 and 4 using tissue microarray. Hum. Pathol. 32, 1392–1397 (2001).
Susaki, E. & Nakayama, K. I. Multiple mechanisms for p27(Kip1) translocation and degradation. Cell Cycle 6, 3015–3020 (2007).
Padar, A. et al. Inactivation of cyclin D2 gene in prostate cancers by aberrant promoter methylation. Clin. Cancer Res. 9, 4730–4734 (2003).
Guo, Y., Sklar, G. N., Borkowski, A. & Kyprianou, N. Loss of the cyclin-dependent kinase inhibitor p27(Kip1) protein in human prostate cancer correlates with tumor grade. Clin. Cancer Res. 3, 2269–2274 (1997).
True, L. et al. A molecular correlate to the Gleason grading system for prostate adenocarcinoma. Proc. Natl Acad. Sci. USA 103, 10991–10996 (2006).
Fleischmann, A. et al. Prognostic relevance of Bcl-2 overexpression in surgically treated prostate cancer is not caused by increased copy number or translocation of the gene. Prostate 72, 991–997 (2012).
Tomlins, S. A. et al. Integrative molecular concept modeling of prostate cancer progression. Nat. Genet. 39, 41–51 (2007).
Hendriksen, P. J. et al. Evolution of the androgen receptor pathway during progression of prostate cancer. Cancer Res. 66, 5012–5020 (2006).
Bismar, T. A., Dolph, M., Teng, L. H., Liu, S. & Donnelly, B. ERG protein expression reflects hormonal treatment response and is associated with Gleason score and prostate cancer specific mortality. Eur. J. Cancer 48, 538–546 (2012).
Furusato, B. et al. Mapping of TMPRSS2-ERG fusions in the context of multi-focal prostate cancer. Mod. Pathol. 21, 67–75 (2008).
Wang, J., Cai, Y., Ren, C. & Ittmann, M. Expression of variant TMPRSS2/ERG fusion messenger RNAs is associated with aggressive prostate cancer. Cancer Res. 66, 8347–8351 (2006).
West, A. F., O'Donnell, M., Charlton, R. G., Neal, D. E. & Leung, H. Y. Correlation of vascular endothelial growth factor expression with fibroblast growth factor-8 expression and clinico-pathologic parameters in human prostate cancer. Br. J. Cancer 85, 576–583 (2001).
Erbersdobler, A. et al. Prognostic value of microvessel density in prostate cancer: a tissue microarray study. World J. Urol. 28, 687–692 (2010).
Serrano, M. Cancer regression by senescence. N. Engl. J. Med. 356, 1996–1997 (2007).
Porten, S. P. et al. Changes in prostate cancer grade on serial biopsy in men undergoing active surveillance. J. Clin. Oncol. 29, 2795–2800 (2011).
Ross, H. M. et al. Do adenocarcinomas of the prostate with gleason score (gs)<=6 have the potential to metastasize to lymph Nodes? Am. J. Surg. Pathol. 36, 1346–1352 (2012).
Eggener, S. et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J. Urol. 185, 869–875 (2011).
Haffner, M. et al. Tracking the clonal origin of lethal prostate cancer. J. Clin. Invest. 123, 4918–4922 (2013).
Cooperberg, M., Simko, J. & Falzarano, S. Development and validation of the biopsy-based genomic prostate score (GPS) as a predictor of high grade or extracapsular prostate cancer to improve patient selection for active surveillance [abstract 2131]. Presented at the American Urologic Association meeting, San Diego, USA (2013).
Knezevic, D. et al. Analytical validation of the Oncotype DX prostate cancer assay—a clinical RT-PCR assay optimized for prostate needle biopsies. BMC Genomics 14, 690 (2013).
Cuzick, J. et al.Prognostic value of a cell cycle progression signature for prostate cancer death on conservatively managed needle biopsy cohort. Br. J. Cancer 106, 1095–1099 (2012).
Robinson, K. et al. Accurate prediction of repeat prostate biopsy outcomes by a mitochondrial DNA deletion assay. Prostate Cancer Prostatic Dis. 13, 126–131 (2013).
Stamey, T. A. et al. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer 71 (Suppl. 3), 933 (1993).
Wolters, T. et al. A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. J. Urol. 185, 121–125, (2011).
Heath, I. Overdiagnosis: when good intentions meet vested interests--an essay by Iona Heath. BMJ 347, f6361 (2013).
Welch, H. G. & Black, W. C. Overdiagnosis in cancer. J. Natl Cancer Inst. 102, 605–613 (2010).
Jemal, A., Siegel, R., Xu, J. & Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 60, 277–300 (2010).
Schroder, F. H. et al. Screening and prostate-cancer mortality in a randomized European study. N. Engl. J. Med. 360, 1320–1328 (2009).
Roobol, M. J. et al. Prostate cancer mortality reduction by prostate-specific antigen-based screening adjusted for nonattendance and contamination in the European Randomised Study of Screening for Prostate Cancer (ERSPC). Eur. Urol. 56, 584–591 (2009).
Hugosson, J. et al. Mortality results from the Gotebörg randomised population-based prostate-cancer screening trial. Lancet Oncol. 11, 725–732 (2010).
Crawford, E. D. et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. J. Clin. Oncol. 29, 355–361 (2011).
Kwiatkowski, M., Klotz, L., Hugosson, J. & Recker, F. Comment on the US Preventive Services Task Force's draft recommendation on screening for prostate cancer. Eur. Urol. 61, 851–854 (2012).
Payton, S. Prostate cancer: new PSA screening guideline faces widespread opposition. Nat. Rev. Urol. 9, 351 (2012).
Draisma, G. et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J. Natl Cancer Inst. 101, 374–383, (2009).
Klotz, L. et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J. Clin. Oncol. 28, 126–131 (2010).
Bul, M. et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur. Urol. 63, 597 (2013).
Dall'Era, M. A. et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer 112, 2664–2670 (2008).
Khatami, A. et al. PSA doubling time predicts the outcome after active surveillance in screening-detected prostate cancer: results from the European randomized study of screening for prostate cancer, Sweden section. Int. J. Cancer 120, 170–174 (2007).
Carter, H. B. et al. Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins experience. J. Urol. 178, 2359–2365 (2007).
Roemeling, S. et al. Active surveillance for prostate cancers detected in three subsequent rounds of a screening trial: characteristics, PSA doubling times, and outcome. Eur. Urol. 51, 1244–1250 (2007).
Soloway, M. S. et al. Active surveillance; a reasonable management alternative for patients with prostate cancer: the Miami experience. BJU Int. 101, 165–169 (2008).
Hardie, C. et al.Early outcomes of active surveillance for localized prostate cancer. BJU Int. 95, 956–960 (2005).
Patel, M. I. et al. An analysis of men with clinically localized prostate cancer who deferred definitive therapy. J. Urol. 171, 1520–1524 (2004).
Porten, S. P. et al. Changes in prostate cancer grade on serial biopsy in men undergoing active surveillance. J. Clin. Oncol. 29, 2795–2800 (2011).
Popiolek, M. et al.Natural history of early, localized prostate cancer: a final report from three decades of follow-up. Eur. Urol. 63, 428–435 (2013).
Lecornet, E. et al.The accuracy of different biopsy strategies for the detection of clinically important prostate cancer: a computer simulation. J. Urol. 188, 974–980 (2012).
Vargas, H. A. et al. Magnetic resonance imaging for predicting prostate biopsy findings in patients considered for active surveillance of clinically low risk prostate cancer. J. Urol. 188, 1732–1738 (2012).
Krakowsky, Y., Loblaw, A. & Klotz, L. Prostate cancer death of men treated with initial active surveillance: clinical and biochemical characteristics. J. Urol. 184, 131–135 (2010).
Vickers, A. J. Systematic review of pretreatment prostate specific antigen velocity and doubling time for prostate cancer. J. Clin. Oncol. 27, 398–403 (2008).
Loblaw, A. J., Savage, C., O'Brien, M. F. & Lilja, H. Comparing prostate specific antigen triggers for intervention in men with stable prostate cancer on active surveillance. J. Urol. 184, 1942–1946 (2010).
US Department of Health and Human Services. NHI Consensus Development Plan [online].
Esserman, L. & Thompson, I. Solving the overdiagnosis dilemma. J. Natl Cancer Inst. 102, 582–583 (2010).
Ahmed, H. U. et al. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol. 13, 622–632 (2012).
Valerio, M. et al. The role of focal therapy in the management of localised prostate cancer: a systematic review. Eur. Urol. http://dx.doi.org/10.1016/j.eururo.2013.05.048.
Zelefsky, M. J. et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J. Clin. Oncol. 28, 1508–1513 (2010).
Kibel, A. S. et al. Survival among men with clinically localized prostate cancer treated with radical prostatectomy or radiation therapy in the prostate specific antigen era. J. Urol. 187, 1259–1265 (2012).
Nepple, K. G. Mortality after prostate cancer treatment with radical prostatectomy, external-beam radiation therapy, or brachytherapy in men without comorbidity. Eur. Urol. 64, 372–378 (2013).
Barzell, W. E. & Melamed, M. R. Appropriate patient selection in the focal treatment of prostate cancer: the role of transperineal 3-dimensional pathologic mapping of the prostate--a 4-year experience. Urology 70, 27–35 (2007).
Dickinson, L. et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur. Urol. 59, 477–494 (2011).
Moore, C. M. et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur. Urol. 63, 125–140 (2013).
Crawford, E. D. et al. Clinical-pathologic correlation between transperineal mapping biopsies of the prostate and three-dimensional reconstruction of prostatectomy specimens. Prostate 73, 778–787 (2013).
Haffner, J. et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 108, E171–E178 (2011).
National Institute of Clinical Excellence. UK Clinical Guideline Prostate Cancer CG175 [online], (2014).
Numao, N. et al.Usefulness of pre-biopsy multiparametric magnetic resonance imaging and clinical variables to reduce initial prostate biopsy in men with suspected clinically localized prostate cancer. J. Urol. 190, 502–508 (2013).
Arumainayagam, N. et al. Multiparametric MR imaging for detection of clinically significant prostate cancer: a validation cohort study with transperineal template prostate mapping as the reference standard. Radiology 268, 761–769 (2013).
PROstate MRI Imaging Study (PROMIS). Current Controlled Trials [online], (2014).
Siddiqui, M. M. et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur. Urol. 64, 713–719 (2013).
Barzell, W. E. et al. Identifying candidates for active surveillance: an evaluation of the repeat biopsy strategy for men with favorable risk prostate cancer. J. Urol. 188, 762–767 (2012).
Nafie, S., Pal, R. P., Dormer, J. P. & Khan, M. A. Transperineal template prostate biopsies in men with raised PSA despite two previous sets of negative TRUS-guided prostate biopsies. World J. Urol. http://dx.doi.org/10.1007/s00345-013-1225-x (2013).
Ahmed, H. U. et al. Characterizing clinically significant prostate cancer using template prostate mapping biopsy. J. Urol. 186, 458–464 (2011).
Wolters, T. et al. Comparison of incidentally detected prostate cancer with screen-detected prostate cancer treated by prostatectomy. Prostate 72, 108–115 (2011).
Valerio, M. et al. The role of focal therapy in the management of localised prostate cancer: a systematic review. Eur. Urol. http://dx.doi.org/10.1016/j.eururo.2013.05.048.
US National Library of Medicine. ClinicalTrials.gov [online]
Kazer, M. W., Psutka, S. P., Latini, D. M. & Bailey, D. E. Jr. Psychosocial aspects of active surveillance. Curr. Opin. Urol. 23, 273–277 (2013).
Sundi, D. et al. African American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: should active surveillance still be an option for them? J. Clin. Oncol. 24, 2991–2997 (2013).
Duffield, A. S., Lee, T. K., Miyamoto, H., Carter, H. B. & Epstein, J. I. Radical prostatectomy findings in patients in whom active surveillance of prostate cancer fails. J. Urol. 182, 2274–2278 (2009).
Gawende, A. Two hundred years of surgery. N. Engl. J. Med. 366, 1716–1723 (2012).
van den Bergh, R. C. et al. Outcomes of men with screen-detected prostate cancer eligible for active surveillance who were managed expectantly. Eur. Urol. 55, 1–8 (2009).
Onik, G. et al. "Male lumpectomy": focal therapy for prostate cancer using cryoablation. Urology 70 (Suppl. 6), 16–21 (2007).
Ellis, D. S., Manny, T. B. Jr & Rewcastle, J. C. Focal cryosurgery followed by penile rehabilitation as primary treatment for localized prostate cancer: initial results. Urology 70 (Suppl. 6), 9–15 (2007).
Murat, F. J. et al. Mid-term results demonstrate salvage high-intensity focused ultrasound (HIFU) as an effective and acceptably morbid salvage treatment option for locally radiorecurrent prostate cancer. Eur. Urol. 55, 640–647 (2009).
Truesdale, M. D. et al. An evaluation of patient selection criteria on predicting progression-free survival after primary focal unilateral nerve-sparing cryoablation for prostate cancer: recommendations for follow up. Cancer J. 16, 544–549 (2010).
Ward, J. F. & Jones, J. S. Focal cryotherapy for localized prostate cancer: a report from the national Cryo On-Line Database (COLD) Registry. BJU Int. 109, 1648–1654 (2012).
Bahn, D. et al. Focal cryotherapy for clinically unilateral, low-intermediate risk prostate cancer in 73 men with a median follow-up of 3.7 years. Eur. Urol. 62, 55–63 (2012).
Ahmed, H. U. et al. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol. 13, 622–632 (2012).
Nguyen, P. L. et al. Updated results of magnetic resonance imaging guided partial prostate brachytherapy for favorable risk prostate cancer: implications for focal therapy. J. Urol. 188, 1151–1156 (2012).
Barret, E. et al. Morbidity of focal therapy in the treatment of localized prostate cancer. Eur. Urol. 63, 618–622 (2013).
Acknowledgements
M.E. receives research support from the UK National Institute of Health Research UCLH/UCL Biomedical Research Centre, London, UK.
Author information
Authors and Affiliations
Contributions
Both authors researched the data for the article, provided a substantial contribution to discussion of the content, wrote and reviewed the manuscript before submission and after peer review.
Corresponding author
Ethics declarations
Competing interests
M.E. has received research awards, has provided consultancy advice and has delivered lectures to the following companies: AngioDynamics, GSK, SonaCare Medical and Sanofi. He is also medical director to Nuada Medical Ltd. L.K. declares no competing interests.
Rights and permissions
About this article
Cite this article
Klotz, L., Emberton, M. Management of low risk prostate cancer—active surveillance and focal therapy. Nat Rev Clin Oncol 11, 324–334 (2014). https://doi.org/10.1038/nrclinonc.2014.73
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2014.73
This article is cited by
-
Ribonuclease 4 is associated with aggressiveness and progression of prostate cancer
Communications Biology (2022)
-
Prostate cancer
Nature Reviews Disease Primers (2021)
-
Making a case “against” focal therapy for intermediate-risk prostate cancer
World Journal of Urology (2021)
-
Diagnostic and prognostic potential of the proteomic profiling of serum-derived extracellular vesicles in prostate cancer
Cell Death & Disease (2021)
-
Meta-analysis of predictive models to assess the clinical validity and utility for patient-centered medical decision making: application to the CAncer of the Prostate Risk Assessment (CAPRA)
BMC Medical Informatics and Decision Making (2019)