Discussion
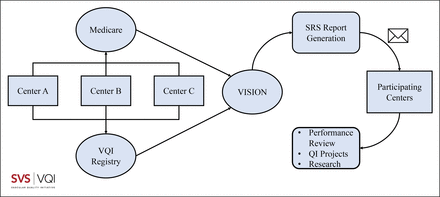
Tracking patient outcomes and quality of care is a long-standing effort that many registries have sought to address. These efforts most often use a single data source (eg, patient chart review, billing data). While such efforts are commendable, these methods—when used in isolation—possess inherent limitations in either the granularity of clinical information available, or the ability to follow patients across space and over larger swaths of time. Here, we outline a hybrid-data approach used to generate reports on centre-specific outcomes.
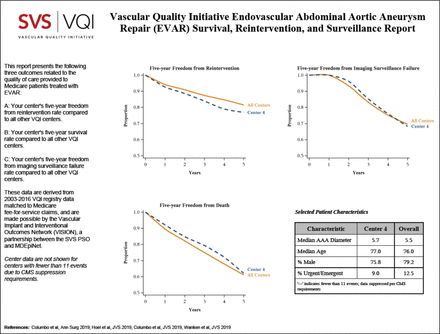
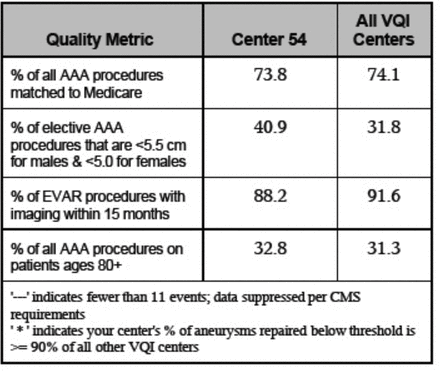
VQI SRS reports are a tool leveraging hybrid data sources to facilitate quality improvement within vascular surgery. The long-term follow-up offered by these reports represents a key strength of this approach. For example, patients being lost to follow-up (relocation, death, etc) has traditionally limited the collection of any further data from a given patient. VISION’s linkage to Medicare claims, however, allows for ongoing capture of pertinent clinical events (reintervention, amputation, death, etc) across multiple centres. In addition, the SRS reports can serve as a tool for a centre to compare its performance to other VQI participants. By easily visualising where one’s centre lies among peers on key performance indicators, it becomes easier to highlight specific areas for improvement. The quality metrics reviewed also offer insights into patient demographics which can be related to how well centres are conforming to various guidelines. For example, AAA metrics such as that seen in figure 3 demonstrate what per cent of elective AAA surgeries at a given centre are being performed below consensus size thresholds, as well as how well a centre meets recommended postoperative imaging surveillance guidelines.
The SRS reports are not without limitations. Due to CMS suppression requirements, reports are only available to centres that perform a sufficient number of cases with Medicare patients. In addition, the specificity could be improved with more granular data on patient demographics and device data, however, further disaggregation at the centre level would reveal smaller numbers which is not permitted by CMS. Another limitation of the SRS reports is the time lag of the necessary data due to the time needed for claims data cleaning, application, and processing; For example, only data up to 2018 was fully available for inclusion in the 2021 SRS reports.
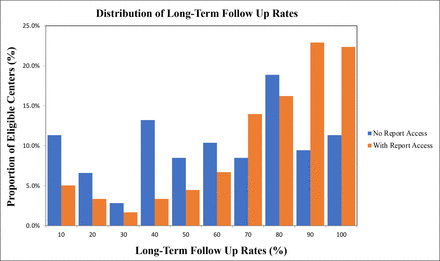
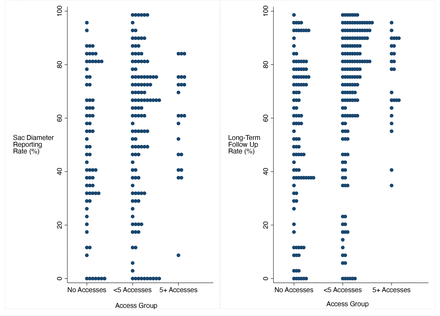
Looking ahead, the SRS reports could be improved by focusing on centre adoption and additional reporting metrics. Regarding centre use of the SRS reports, our preliminary study of report access revealed that ~59% receiving centres actually opened the report. We hope with improved communications and ease of access to these reports we might be able to improve the uptake of these SRS reports. From a content standpoint we hope to improve the SRS reports by incorporating implant data. As a specialty heavily reliant on various devices such as grafts and stents, device-level outcomes could offer insights to not only vascular surgery centres, but to additional stakeholders such as device manufacturers, the Food and Drug Administration (FDA), and even patients themselves. For example, similar reports could be created to monitor device performance rather than centre-specific performance. If a specific device reveals a pattern of early failure, that could have implications for surgeon device selection, manufacturer recalls and FDA review. In the future, we also hope to further refine the reports with more granular additions such as sex-specific metrics.
Looking ahead, new or existing registries could adopt similar methods to deliver centre-specific reports. Importantly, the methods outlined for creating the SRS reports are not specific to vascular surgery and could be employed in other specialties, surgical or otherwise. Some potential uses for these methods could include assessing compliance with various evidence based guidelines or disparities in treatment based on sex, race/ethnicity, geography, etc. In addition, within the surgical community, registries have often focused on shorter-term outcomes such as in-hospital complications or 30-day mortality. Our methods show how leveraging Medicare claims data facilitates tracking patient outcomes over longer periods of time, and we believe that better understanding the longevity of our interventions and implantable devices is, and will continue to be, of growing importance.