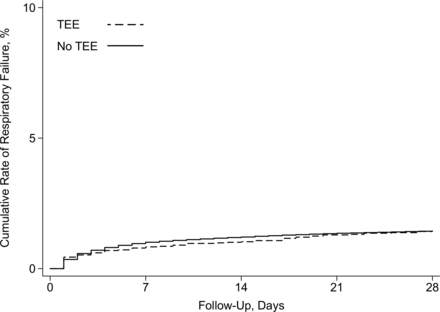
Discussion
In this IDEAL phase 4 study using a large sample of Medicare beneficiaries who had ischemic stroke and TIA, we found that TEE was not associated with an increased risk of respiratory failure after adjustment for demographics, comorbidities, stroke versus TIA diagnosis, and acute stroke treatments. In stratified analyses, this finding was similar in patients with stroke versus TIA. Our results were unchanged across multiple sensitivity analyses, including exclusion of patients with infective endocarditis and patients undergoing cardioversion, exclusion of patients who died during hospitalization or were discharged to hospice, limiting the outcome to respiratory failure requiring tracheostomy, including a TEE–calendar year interaction term to determine whether the relationship between TEE and respiratory failure changed over time, and including patients who experienced respiratory failure on the same day as TEE. Notably, the total incidence of respiratory failure was lower than the incidence reported in prior studies.17 This is most likely because patients requiring intubation on the day of admission were not included in our analysis as we were focused on respiratory failure occurring at least 1 day after TEE.
Data are scarce on the safety of TEE in regard to respiratory failure in the context of stroke or TIA. Several factors may increase risk of aspiration after TEE for patients who had stroke and TIA, including older age and frailty, dysphagia,18–21 use of sedating medications,22 and direct injury from probe manipulation during the procedure.23 Although prior studies have suggested TEE is generally safe and well tolerated in general cardiac populations,24–30 safety data in stroke and TIA have only been sparsely reported in relatively small, single-center studies.5 Recent evidence from studies not specifically enrolling patients who had stroke or TIA suggests that the risk of complications associated with TEE may be higher than previously suggested.23 31 In this context, our results provide novel findings suggesting that the use of TEE for routine, community-onset stroke or TIA may not be associated with an increased risk of respiratory complications.
Our study has several limitations that should be considered when interpreting our results. First, our results were limited to patients with an admission diagnosis of stroke or TIA, and because we lacked data on the exact timing of strokes occurring during the hospitalization we could not examine the association between TEE and respiratory failure in patients who experienced in-hospital stroke or TIA. Second, our variables were defined using diagnosis and procedure codes, which carry a risk of misclassification. Third, we lacked data regarding the type, dosage, and depth of sedation in patients who underwent TEE. Fourth, the etiology of respiratory failure is often multifactorial and thus our results may be prone to residual confounding and selection bias. Fifth, all patients included in our study were at least 65 years of age and the results may not be generalizable to all age groups. Sixth, we do not have data on do-not-intubate orders, other requests for limitation of care, or the overall goals of care. It is possible that our definition of respiratory failure missed cases of respiratory failure which were not treated with invasive means because of more conservative goals of care, and such patients may also have been less likely to undergo other invasive procedures such as TEE. On the other hand, such patients with respiratory failure would presumably not have survived or been discharged to hospice, and our findings were unchanged in a sensitivity analysis excluding patients who died or were discharged to hospice. Seventh, for patients who experienced respiratory failure on the same day as TEE, we were unable to specify which event occurred first. Conservatively, we did not count TEE performed on the same day as respiratory failure to avoid results driven by cases in which TEE may have been performed in patients who were already intubated; however, this approach may also carry a risk of selection bias by excluding cases in which respiratory failure occurred during or immediately after TEE. Eighth, we did not have data on TEE operator technique or experience or how these variables may have changed over time. These unmeasured variables could confound the relationship between TEE and respiratory failure, although we found no evidence that this relationship changed by calendar year. Finally, a complete accounting of the risks and benefits associated with TEE was not possible with the data available as we do not know the full clinical indications for TEE in each case or the yield of the TEE studies.
In conclusion, we found that TEE was not associated with an increased risk of acute respiratory failure among older patients who had acute ischemic stroke or TIA. These findings may help clinicians weigh the potential risks and benefits of TEE for evaluation of ischemic stroke.