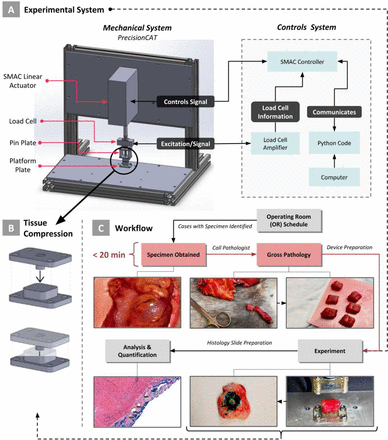
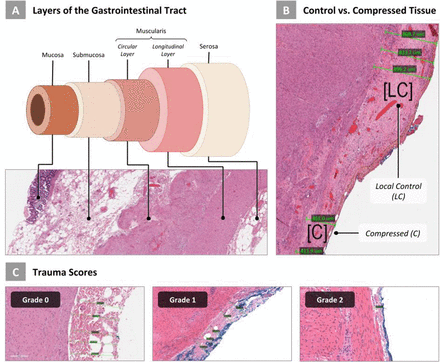
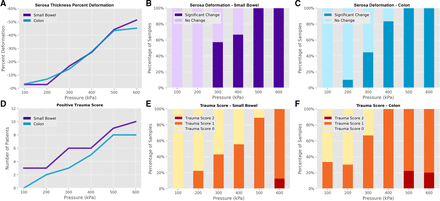
Introduction
Laparoscopic graspers are used extensively in minimally invasive surgery, primarily to lift and mobilize delicate anatomical tissues for better visualization and access.1 However, serious iatrogenic complications from the improper use of laparoscopic graspers in bowel surgery can occur and include: bowel perforation, serosal tears and postoperative adhesion formation.2 Bowel perforation is an especially severe complication because it is associated with a high morbidity and mortality rate (as high as 3.6%).3 4 Tang et al studied the removal of the gallbladder, a routine gastrointestinal laparoscopic procedure. When analyzing consequential and inconsequential errors committed using various surgical instruments, the error probability of holding graspers and dissecting graspers were higher than those of other surgical tools such as the electrosurgical hook knife. They found that perforation of the gallbladder and bleeding from liver injury were mainly caused by the use of excessive force or dissection in the wrong tissue planes.5
Careful evaluation of these statistics is especially pertinent in light of the fact that 100 patients a day die from iatrogenic injuries in US hospitals, with 40% of these injuries occurring in the operating room.6 7 In Canada, Baker et al, studied adverse events occurring in hospitals across five different provinces. They found that 7.5% of all patients admitted to acute care hospitals experienced one or more adverse event(s), with 51.4% of all adverse events arising from surgery. They judged that 36.9% of these adverse events were highly preventable.8
Unfortunately, there has been a dearth of studies that attempt to quantify the interaction of the grasper-tissue interface at a histological level to quantify which load forces tissue injuries occur at. This topic is particularly important to explore, because researchers have found that the handle and tip forces in laparoscopic graspers differ significantly from conventional graspers used in “open-approach” surgeries, which can lead to inappropriate force magnitudes and tissue damage.9 10
Complex mechanical response of tissue to compression
It is challenging to accurately quantify and model biological soft tissues’ multifaceted and complex behavior in response to the compressive force exerted by laparoscopic graspers. The mechanical response of tissue is based on two factors: (a) the inherent mechanical properties of that tissue and (b) the environmental loading characteristics it is subjected to. The small and large bowel are composed of multiple tissue layers. Within each layer, different fibers are distributed according to specific spatial orientations, which creates a strongly anisotropic configuration where measured properties varies along its different axes.11
Grasper jaw geometry and stress on tissues
Laparoscopic graspers have jaws traditionally made from stainless steel due to its durability and ease of sterilization. The main disadvantage of using metal is that metal is a much stiffer material than delicate gastrointestinal tissues and as such, compressing tissue with metal graspers can cause damage at the cellular level (such as mechanical destruction of the cell membrane or nucleus) or tissue level (such as rupture of muscle fibers or ischemia from the destruction of blood vessels).12 Graspers also come in a variety of jaw geometries and teeth profiles such as straight or flared, fenestrated with waves or solid and single or dual action, which contribute both to their function and damage potential. For example, when a grasper’s jaw is serrated, increasing the size of the teeth will help prevent slippage but also causes more damage to tissue.13 Cheng and Hannaford investigated this relationship and created both a two-dimensional and three-dimensional finite element analysis study of calculated von Mises stress distributions under compression loads in a grasper and liver tissue model.14 They found that in the two-dimensional plane strain model, that 80% of the stress in the area directly beneath the grasper was over 300 kPa, which is over the damage limit they elucidated in their previous work of 240 kPa for liver tissue.15
Is porcine tissue an accurate surrogate for human tissues?
Previous studies exploring grasper jaw and tissue interactions have mostly centered on porcine tissue studies. This is due to the vast logistical and ethical challenges involved in human tissue experimentation and the previous assumption that porcine tissues are close enough to human tissues to be a surrogate model. Christensen et al’s work with porcine and human bowel tissues casts doubt on this assumption; however, as they found that human tissues were stronger, stiffer and less compliant than porcine tissue. Porcine tissue was able to stretch almost twice as much as human bowel tissue (with an elastic modulus of 1.83 MPa and 5.18, respectively), while human bowel tissue had a higher ultimate average strength (0.58 MPa compared with 0.87 for human tissues).16 Heijnsdijk et al also found that the inter-individual variability in perforation forces is quite large and that bowel strength could differ by a factor of two between patients.17
Establishing safe tissue force boundaries in humans with a histopathological analysis
This study aims to investigate the relationship between grasper jaw forces and human small and large bowel (colon) tissues. These tissue types were specifically chosen for two reasons: first, they are the most clinically relevant in relation to repeated grasp injury and second, bowel is one of the most delicate tissues in the human body. van der Voort et al4 found that the overall incidence of laparoscopy-induced bowel injury was 0.36%. The small intestine was most frequently injured (55.8%), followed by the large intestine (38.6%).3
To the authors’ knowledge, this will be the first study to investigate the upper limit of force by laparoscopic graspers in human tissues with a histological analysis of cellular damage. A histological model was chosen to objectively and quantifiably understand how the intestinal tissue structure is microscopically affected as a result of mechanical loading. These data will be important as we move into an age of “smart surgical tools” that can quantify tool-tissue force interactions.