Discussion
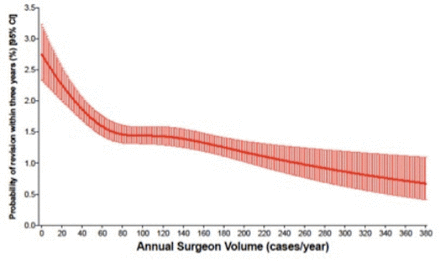
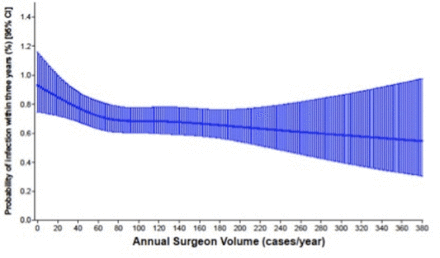
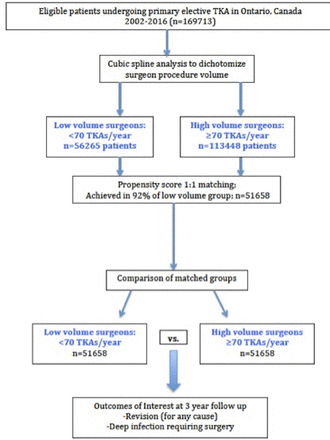
The model used in this study indicated that as surgeon volume increased, the risks for complications decreased. We observed an inflection point at 70 TKAs/year, after which the rate of decrease in the risks for complications leveled off. After performing a propensity score match for surgeon volume, which also controlled for surgeon experience, we found that in patients operated on by surgeons with annual volumes <70 cases, the relative risk for revision (for any cause) increased by 31% (2.23% (95% CI 1.39 to 3.07) vs 1.70% (95% CI 0.85 to 2.55)), and the relative risk of deep surgical infection requiring surgery increased by 18% (1.29% (95% CI 0.44 to 2.14) vs 1.09% (95% CI 0.24 to 1.94)). The findings of this study indicate that for surgeons performing <70 primary TKAs per year, regardless of previous experience, there is an increased likelihood for these two complications.
Use of the above approach is lacking in other studies which define volume categories instead. One such study used stratum-specific likelihood ratio analysis, a method of analyzing receiver operating characteristic curves to show that there is a significant decrease in 90-day complication and 2-year revision rates for surgeons in higher volume categories (60–145 TKAs per year and >146 TKAs per year) vs those in lower volume categories (0–12, 13–59 TKAs per year).26 A criticism of the aforementioned study is that it does not take account of changes in procedures and practices over time,27 something which the cubic spline technique is able to account for, as it is a dynamic assessment of a surgeon’s practice, meaning that each individual surgeon can have different volumes across the study period.
The threshold of 70 primary TKAs per year is higher than previously reported for total hip arthroplasty (THA) (35 cases per year28). A possible reason for this difference is that the rates of early complications after TKA are generally a little lower than after THA; as such, a higher volume threshold is required to observe a difference in outcomes. The THA study complications included dislocation, periprosthetic fracture, and a broader definition for infection. The early complications of venous thromboembolism and death within 90 days were also included in that study.
Many patients do not have the means or social support to travel to high volume TKA providers from rural areas. Having a healthcare policy that restricts provision of TKAs to only high volume surgeons can have the effect of restricting access to care. Research should be focused on improving quality of care and ensuring that it is uniform across surgeons with varying volume.27 This has significant cost implications in terms of training, revalidation and continuing professional development.
Through the use of restricted cubic splines, we found that there was a noticeable decrease in likelihood of revision (for any cause) and deep infection requiring surgery, as the surgeon’s yearly primary knee arthroplasty volume increased; however, the relationship was not linear. While the relative improvement in risk of revision (for any cause) and deep infection requiring surgery with increasing surgeon volume attenuated after this point, there continued to be a downward trend in the risks for these two complications, indicating that increased surgeon volume continues to have a beneficial impact, although one that is less pronounced.
The cumulative risk of revision for TKAs in the National Joint registry for England and Wales ranges from 1.53% for cemented TKAs, to 1.80% for hybrid TKAs, and 2.09% for uncemented TKAs, within 3 years following surgery.29 The absolute difference in revision rates between these implant designs is of similar magnitude (0.56) to the difference between low and high volume surgeons found in the current study (0.53).
There are limitations to the work we have presented. First, we did not have any information on patient-reported outcomes. Second, we are unable to report a subgroup analysis based on the indications for revision TKA, nor assess center effects in the modelling we have undertaken. We were also unable to control for technical aspects of the procedure, for example, severity of pre-existing deformity, the need for adjunctive soft tissue releases, or length of surgery, factors which have been linked with complication following TKA.30 With regard to the analysis performed, there is a risk of introducing bias with propensity score matching.31 In this study, however, we have matched more than 90% of the cases performed by low volume surgeons, suggesting a representative sample, and standardized differences for all relevant measured confounders were under 10% after matching.
This study was conducted in Ontario, Canada where it is standard practice for healthcare organizations (hospitals or hospital networks) to standardize implants for use by their surgeons. For the vast majority of surgeons therefore, implant selection would be equivalent irrespective of volume. We accept that having the detail of the types of brands may help define if there is an advantage for low volume surgeons using the best performing implants but the converse could also be the case. Unfortunately, this detail is not something we have available in our database to provide. There is no role for private practice in the provision of joint replacement; hence, all practicing surgeons are accounted for in the available data set. Surgeons registered with the College of Physicians and Surgeons of Ontario are not allowed to work across two provinces. As such, we are confident that the surgeon volumes captured in the study are not an underestimate, but we also accept that we are unable to account for the unlikely movement of surgeons in and out of the Ontario system during the period of the study.
Further research, potentially using data sources that capture this information, is recommended to confirm or refute these hypotheses. With regard to deep infection requiring surgery as an outcome, we have used a limited definition (cases requiring irrigation and debridement, liner exchange or spacer insertion), meaning that we have not captured instances of superficial surgical site infection (SSI). It has recently been demonstrated that increased surgical duration is associated with a higher risk for infection following TKA32 and this is a plausible mechanism linking low volumes and infection following TKA. Infection should be viewed as a spectrum from acute to chronic.33 We define an infection in our database as one that is serious enough to warrant an additional intervention such as spacer insertion or wound irrigation and debridement. The rate of superficial SSIs that are managed primarily by surgeons or primary care providers is difficult to quantify and this information is not available in our database.
This paper and the description of the study methodology provide an opportunity for a similar type of analysis to be carried out in different geographical locations/settings as it may be inappropriate to apply a number obtained from one region to another. This is especially important as the median numbers performed per year, are different between countries (median number of TKAs per surgeon in UK (n=36)29; in the USA median number of TKAs per surgeon (n=23)34).