Discussion
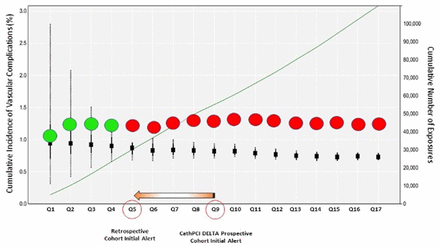
The CathPCI DELTA study was designed to assess the feasibility of prospective, active safety surveillance of a large clinical data repository to support safety outcomes monitoring of post-market medical devices. We performed a large, propensity score matched, safety surveillance study of a national cardiovascular clinical registry and identified a safety alert for vascular complications associated with use of the Mynx VCD. In the current analysis, we were able to improve safety signal detection time by 12 months through inclusion of 36,693 retrospective Mynx VCD cases over an additional analysis period of 18 months. This analysis confirmed a higher risk of vascular complications, access site bleeding, and transfusion requirement among a larger cohort of Mynx VCD recipients compared with those treated with alternate VCDs.
The RR for vascular complications associated with the Mynx VCD was 1.63 (95% CI 1.50 to 1.79; p<0.001) with an absolute event rate of 1.20% versus 0.73% with alternate devices. Had the device of interest been recalled after identifying the first safety alert in this study, between 952 and 1035 additional vascular complications may have been avoided.
The appropriate actions following detection of a safety alert have not been fully established. Given the large sample sizes available in national data registries, alerts that achieve statistical significance should be interpreted in the context of their clinical significance. Depending on estimations of absolute event rates, risk thresholds that prompt additional action should be prespecified. The regulatory actions that follow should depend not only on risk estimates, but on the gravity of the clinical outcome event of interest, and the confidence in this signal. For example, a safety signal indicating higher rates of death associated with the use of a medical device may prompt more immediate action at a lower risk ratio with lower confidence. The specific actions that follow should integrate several variables including the frequency of events, seriousness of safety events, and necessity of using the device in consideration. Actions might be limited to manufacturers simply considering device modifications or improving education regarding the use of a specific device or may extend to withdrawal or recall of a device if serious safety concerns are demonstrated.
This study specifically focused on safety of arterial VCDs which were initially brought to market in 1996 in the USA to support safe and expedient hemostasis following femoral arterial access for PCI. Several devices are currently available, and differ with respect to the mechanical, pharmacological, and biomaterial components used. Most have been approved through small pre-market studies, and no data currently supports an improvement in rates of post-procedural bleeding or other complications with their use.21 However, given the improvement in patient comfort compared with prolonged manual compression, and significant improvement in time to achieve hemostasis, VCDs remain widely available and are commonly used. While VCDs offer a convenient solution for arterial hemostasis, the use of such devices, and new devices, should be held to rigorous standards with little tolerance for excess in adverse event rates given their non-essential role in most PCI procedures.
This study has several important limitations. Safety signals, once identified, should be interpreted with caution. We performed robust risk adjustment through the use of propensity score matching. However, the possibility of residual confounding cannot be excluded. Additionally, the variables included in the propensity score matched analysis are limited to those available in the CathPCI data collection tool. Additional anatomic variables such as arterial puncture site, which may contribute to residual confounding, were not available in the CathPCI Registry. Finally, study endpoints were limited to in-hospital events available in the Cath PCI registry.
This study demonstrates the feasibility of automated surveillance within a large, high quality data sources to support post-market monitoring of medical devices. Among existing medical devices, using prospective active surveillance methods, augmented by retrospective registry data may improve early identification of safety differences. For novel medical devices, surveillance should begin early in the device life-cycle to allow rapid and actionable safety alert identification. Overall, our analysis demonstrates that prospective, active surveillance within a large clinical registry is feasible to detect safety differences among commonly used cardiovascular devices.