Discussion
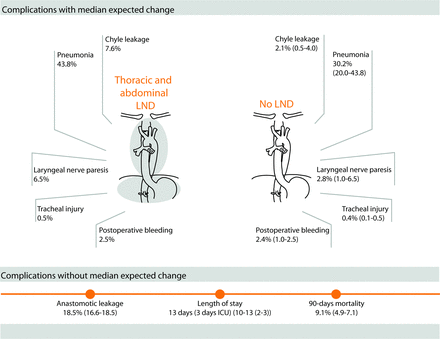
The results of this case vignette survey showed that the routine extent of LND varies considerably between experts worldwide. Guidelines for LND are not always followed; especially the aortopulmonary window LNs and paratracheal LNs are not routinely dissected. Furthermore, the majority of participants would still perform thoracic and abdominal LND even if it is assumed that a hypothetical 100% accurate imaging test did not detect any LN metastases. If LND was omitted, a change in the percentage of chyle leakage, pneumonia and laryngeal nerve paresis was expected, as well as a 60 min shorter operative time. If a diagnostic imaging test was available that could detect the presence of LN metastases after nCRT, a minimum sensitivity of 92% and a specificity of 90% were recommended to personalise LND based on the diagnostic test.
Strength and limitations
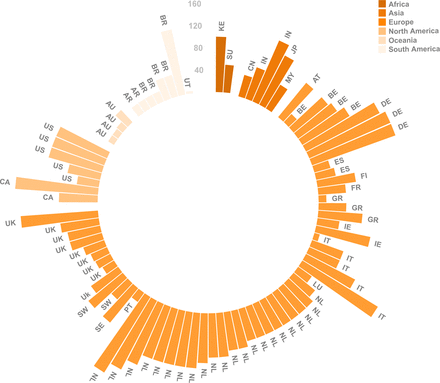
This is the first study investigating the routine extent of standard LND and additionally resected LN stations in case of suspicious LN metastases. Based on worldwide expert opinion, we provided an overview of the implications of personalising LND in oesophageal cancer surgery. Some potential limitations should also be mentioned. First, the response rate was limited for some continents, especially Africa and Oceania, and the results of this survey might therefore not reflect a broader view from these geographical areas. Second, we provided information about the presence of thoracic or abdominal LN metastases in the cases, but the precise location, subtype (adenocarcinoma or squamous cell carcinoma) and number of these metastases was not specified. This might have resulted in different interpretations and answers, since experts could have envisioned the case differently (eg, only one LN metastasis vs multiple LN metastases). However, in this way, we were able to provide an overview of the expert opinion on omitting LND, while differences in practice had little influence on our results. Third, some questions would not likely occur in clinical practice. On the other hand, these theoretical cases provide useful information about adherence to the guidelines for the purpose of policy decisions.
Variation in current practice
The results show considerable variation in current extent of LND as performed by oesophageal surgeons worldwide. Routine two-field LND as recommended in the AJCC guidelines is not routinely performed by all experts, which could limit LND accuracy. Experts indicate multiple reasons for omitting particular LN stations, for example, because of technical difficulties, increased morbidity or a low risk of LN metastases in a particular area.18 Despite a few studies describing the pattern of LN metastases in oesophageal carcinoma,19–21 the distribution of LN metastases has not yet been described in large series. Moreover, it is known that nCRT significantly modifies location and distribution of LN metastases.22 The balance between oncological value (accuracy of LND) and morbidity is therefore unclear. Currently, a worldwide prospective study (TIGER study, distribution of LN metastases in oesophageal carcinoma)15 evaluates the distribution of LN metastases in patients with resectable oesophageal carcinoma. This may lead to new global guidelines for LND.
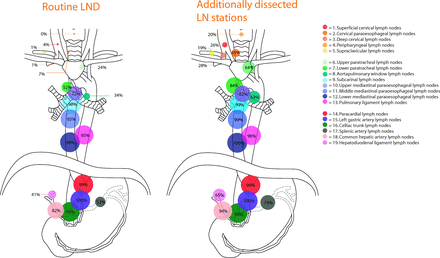
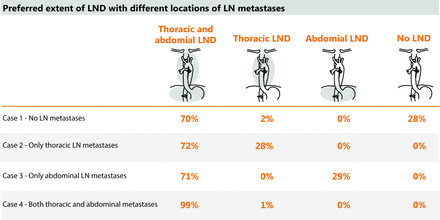
Besides variation in routine LND, participants seem more willing to extend the LND in case LN metastases are found than to omit LND in case no LN metastases are present according to the hypothetical test. In case of a cervical LN metastases, approximately half of the experts would perform a cervical, thoracic and abdominal LND. This is a surprising result, since the cervical LN stations are not part of routine clinical practice for the majority of surgeons (figure 2). In the most recent AJCC guidelines, however, the lower cervical paratracheal, cervical perioesophageal level VI and VII LN stations are also considered to be locoregional LN stations, thereby justifying extension of the LND for the presence of cervical LNs in these particular stations.
Clinical implications
Experts indicate that omitting LND could have advantages in terms of fewer complications and shorter OR time, thereby improving quality of life and reducing costs. Currently, it is unknown if these advantages will weigh up to the possible risks of omitting LND when a diagnostic test is not 100% accurate. Recently, two phase III trials were initiated where watchful waiting is compared with standard surgery for patients with complete response of the tumour and LNs after nCRT.23 24 In these trials, the importance of an accurate diagnostic test after nCRT is crucial as patients with (micro) LN metastases could easily be missed, resulting in unjustified omission of surgery. Results of these trials are therefore of high interest to evaluate the consequences of unjustified omission of surgery.
The implications of accurate LN staging after nCRT will likely have a greater impact than solely omitting LND. For example, surgical approaches with a limited LND, for example, transhiatal oesophagectomy, might be more beneficial in patients without mediastinal LN metastases, given the lower risk of postoperative pulmonary complications. Furthermore, less invasive surgical techniques might prevent even more (functional) morbidity, for example, active surveillance in complete responders after nCRT.
This survey showed reluctance to omit LND despite the absence of LN metastases according to the hypothetical test. Experts mentioned disbelief in the accuracy of the test to detect micrometastases and missing the opportunity to perform LND (as reoperation is often not an option) as reasons not to omit LND. This is not surprising, since current imaging techniques have a relatively low accuracy and a recent meta-analysis showed a higher number of LN dissected during oesophagectomy resulted in better overall survival in this patient group.5 6 25 26 Therefore, further explorative studies to assess the potential impacts of selecting patients for LND, as well as studies into promising diagnostic tests that could accurately detect LN metastases, are crucial to eventually, in case of encouraging results, translate surgical practice into a more personalised approach.
In conclusion, there is no consensus on the extent of LND after nCRT. Oesophageal surgeons seem more willing to extend LND if LN metastases are found rather than omit LND in case no LN metastases are identified. The majority of oesophageal surgeons expect to reduce morbidity and OR time when LND is omitted.