Discussion
Radical prostatectomy or radiation therapy are well-established methods of treating CaP. A high rate of cancer control is achieved, but at the cost of significant functional effects, especially erectile dysfunction. Two contemporary publications suggest that erectile dysfunction remains problematic. Published data from well-designed prospective trials in the UK (ProtecT)27 and the USA (CAESAR)1 demonstrate erectile dysfunction rates of 50%–70% at 12 months after prostatectomy. A report from a single center of excellence in the USA, Memorial Sloan-Kettering Cancer Center, is largely confirmatory (27% potency), despite the use of intracavernosal injections, refinements in nerve sparing, and adoption of robotics.2
The results are quite different in men undergoing simple prostatectomy or prostate-sparing radical cystectomy. Several series report a potency rate of about 90%. This suggests that a key to preserving potency may lie in preserving the “prostatic capsule”,28–30 where the network of erectogenic nerves is located.21 22 In this regard, focal HIFU, which spares at least 50% of the prostate,10–12 has shown promise. The seminal work by Ahmed and colleagues reported a potency rate of 89% at 12 months and a continence rate of 100%.10 Other studies on focal therapy (HIFU and others) have corroborated their findings.11 13 31
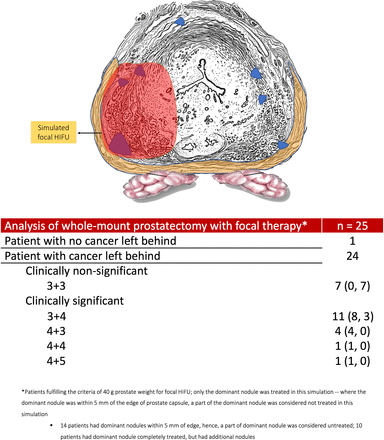
There remains a concern regarding the oncological efficacy of the procedure, however. A multicenter 5-year follow-up of 505 men with intermediate risk CaP who underwent focal HIFU reported a cancer-specific survival of 100%,12 but a retreatment rate of 27% and a positive biopsy rate of 25% in men who were re-biopsied. Another single-institution study of 150 men (n=132, Gleason 7) showed residual cancer in 81% of men who underwent confirmatory biopsy and a 25% retreatment rate, despite an attempt to ablate fivefold to 10-fold the lesion volume detected on mp-MRI.13
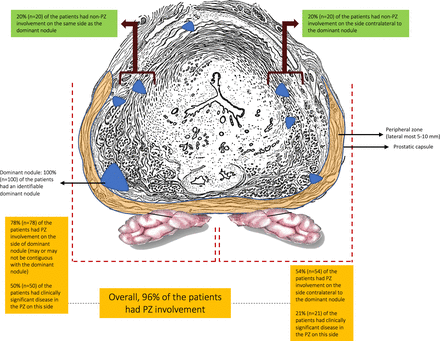
We reasoned that the high anatomical failure rate from focal HIFU may be related to the presence of residual CaP that is below the detection resolution of MRI, currently, 10 mm in diameter or 0.5 cm∧3 in volume. Our preclinical, stage 0, prostatic mapping study was performed to test the validity of this assumption. We found that >70% of men who were candidates for focal or hemiablation therapy harbored low volume clinically significant CaP on analysis of whole-mount specimens. These results are comparable with other reports. Kenigsberg et al in a simulation study of focal HIFU in radical prostatectomy specimens noted that significant cancer (Gleason pattern 4 or above) would have been left behind in 23.7% of the patients who had underwent mp-MRI evaluation.32 Similarly, Elkhoury et al demonstrated that detection rate of Gleason ≥7 cancer via mp-MRI fusion biopsy varies between 47% and 60% in patients with mp-MRI visible lesions, while 15% of clinically significant cancers are invisible.33
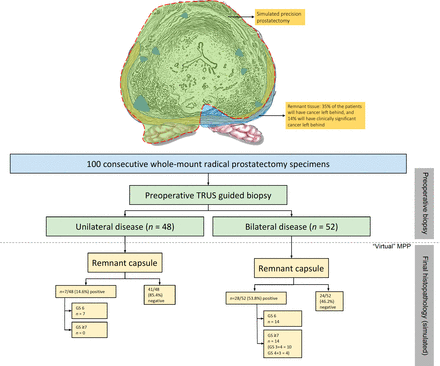
Our analysis allowed us to simulate a novel surgical technique, precision prostatectomy. In this approach, we attempt to remove all prostatic tissue, but for a 5–10 mm rim of prostate capsule on the side contralateral to the dominant lesion. In a “what-if” analysis, if all men had undergone precision prostatectomy, the residual cancer rate would have been 14% (10% Gleason 3+4, 4% Gleason 4+3) while it would have been 68% had they undergone focal HIFU (44% Gleason 3+4, 16% Gleason 4+3, 4% Gleason 4+4% and 4% Gleason 4+5; see figures 2 and 3). Looked at in another way, precision prostatectomy would have spared 86% of men from having whole-gland therapy, with equivalent oncologic control, and a higher erectile function rate than whole-gland therapy. This is probably due to the fact that precision prostatectomy allows for removal of greater than 90% of the prostate with complete removal of the side of the dominant lesion, and removal of the majority of satellite lesions on the contralateral side.
Following IDEAL guidelines, the stage 0 study was followed by a stage 1/2a study of MPP in eight highly selected patients. Precision prostatectomy offers several conceptual benefits over focal/hemiablation. No patient needed to be excluded because of prostate size or the location of the tumor. It is noteworthy that manufacturers’ guidelines suggest limiting focal HIFU to men with prostate volumes less than 40 cc, and to men without apical/anterior tumors, approaching 75% of men in the preclinical study. Further, the treated tissue remains available for detailed pathological or genomic analysis after MPP, while it is destroyed at ablation.
In this development study, we demonstrate that MPP is technically feasible and safe. No patient needed to be converted to radical prostatectomy, or suffered an intraoperative or postoperative complication. Only one modification to technique was made during this series: using intraoperative ultrasonography with a drop-in probe, we were able to measure accurately the volume of residual tissue and confirm that the intent of removing >90% of prostatic tissue was met.
From a functional outcomes standpoint, the patients undergoing precision prostatectomy achieved excellent results, with all eight (100%) patients achieving urinary continence within 4 months of surgery and potency within 12 months.
Evaluation of the oncological effectiveness of focal therapy has been bedeviled by a lack of consensus on what constitutes a PSA failure, an important surrogate for oncological control after radical prostatectomy. After all, a significant portion of the prostate is left behind after focal therapy, and continues to produce PSA. Most investigators are silent about PSA values after focal therapy, but Bass et al found that the PSA nadir was 2–3 ng/mL at 2–3 years of follow-up.13 Using the AUA definition for biochemical recurrence following radical prostatectomy (PSA of 0.2 ng/mL and rising, or a single value of 0.4 ng/mL24), we demonstrated that two out of the eight (25%) patients developed biochemical recurrence, whereas no patient (0%) had a recurrence if we used the ASTRO criteria for recurrence.26 Postoperative biopsy in the two patients with biochemical recurrence (per AUA criteria) showed low-volume, low-risk disease: 1/6 cores of Gleason 3+3 in one patient and 2/6 cores of Gleason 3+3 in the other.
Another important oncological consideration is the evaluation of surgical margins. Information about surgical margins cannot be obtained from ablation studies, where, by definition, no tissue is removed. Further, the use of surgical margins as a meaningful endpoint is debatable: the recent 29-year follow-up of SPCG-4 trial,34 and our prior work,35 suggests that surgical margins matter minimally, if at all, unless patients have extraprostatic disease and high-grade cancer. In line with this, the two patients that had positive surgical margins have not required additional treatment and remain on active surveillance with stable PSAs.
Our study has several limitations. By design, this is a stage 0-1/2a, single-center study evaluating a small number of patients. Hence, conclusions should be drawn with caution. However, the study design is in accordance with recommendations provided by the IDEAL collaboration for surgical innovation. A second limitation is that MRIs were not performed in these men. The reasons for this were partly pragmatic, and partly by intent. At the time that the patients in the stage 0 study were operated on, mp-MRI was not readily available for general clinical use in the USA. And a raison d’etre for the study was to examine the distribution of clinically significant cancers that are smaller than the current detection limit of mp-MRI in a target population that would qualify for focal therapy.
Another limitation of precision prostatectomy is that two out of eight patients had residual cancer on protocol biopsy. Although these patients have not required any further treatment as of the writing of this paper, it suggests that there is room for improvement in selection of the patients. For the reasons explained above, we are not convinced that mp-MRI would eliminate the rate of residual cancer, as this study and others suggest that a substantial proportion of clinically significant tumors are below the detection limits of mp-MRI.18 32 33 36 We are exploring an alternate diagnostic methodology to accurately stage focal therapy candidates preoperatively with 3D transperineal saturation biopsy.37 Biopsies that target specifically the area of the prostate that will be left behind during precision prostatectomy may complement biopsies that target the tissue that is removed, and thus reduce the incidence of missed cancer.
It can also be argued that cancer control requires the treatment of just the index lesion. This conclusion is controversial. Kneppers et al showed that in about 20% of men, lymph node metastases arose from non-index lesions.38 In a more recent study, the Palapattu group demonstrated that, using next generation sequencing, the genetic footprint of MRI invisible lesions was indistinguishable from MRI visible or index lesions.39 Further, the short duration of follow-up and the high incidence of secondary treatment after focal HIFU argue for the development of newer approaches, a better mouse trap, if you will. It seems reasonable to plead that if new procedures are being contemplated, their development should follow a structured pattern, the IDEAL path.
Finally, conclusions about functional superiority are best resolved with randomized clinical trials. We are in the process of doing such a trial. The data reported here merely form a basis of such a trial.